Templar
Hazard to Self
 
Posts: 82
Registered: 17-8-2014
Location: The Sprawl, Titan
Member Is Offline
Mood: No Mood
|
|
isatoic anhydride condensation
Hey guys,
Got a question here, bit lost for reaction mechanics and what not. I am doing some experimentation with novel quinazoline alkaloids, but solubilities
and reactivities of the amide/amine groups have me a bit confused as well.
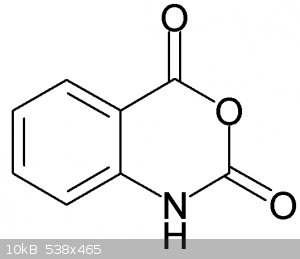
Condensation of isatoic anhydride with o-toluidine would yield 2-Amino-N-(2-methylphenyl)benzamide, not 2,2'-dimethylbenzanilide ?
2 amino n 2 methylphenyl benzamide:
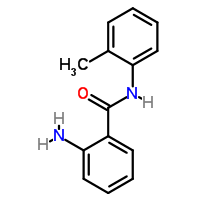
Clearly the isatoic anhydride side ring opens and the carboxylic acid group attached to the benzene ring makes an amine salt with the o-toluidine,
which is then dehydrated to the amide through heat.
According to manhas, Then somehow the amide functionality on the right leaves (possibly as methanamide/formamide?) and the nitrogen attached to the
2nd carbon is replaced with a methyl.
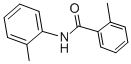
It doesnt make sense to me. Neither can acetylacetone condense to form a ring with only one N atom.
Manhas, A Facile Synthesis of Methaqualone and Analogs, Synthesis 5, 309-10 (1977) (if someone has this, posting it would be great. I cannot get at
it!)
Step 1:
Method A
A mixture of isatoic anhydride (1.6 g, 0.01 mole) and o-toluidine (1,1 g, 0,01 mol) is heated to 120° for 2 hours. The reaction mixture after cooling
is triturated with ether (or dissolve the brown mixture in warm acetone and add water to crash out the crystals). The resulting solid is collected by
suction and recrystallized from a 50:50 mixture of dichloromethane and petroleum ether to give the intermediate aminoamide (2,2'-dimethylbenzanilide):
yield: 1,7 g (75%): m.p. 110°.
A mixture of 2,2'-dimethylbenzanilide (0.5 g, 0.0025 mole), acetylacetone (0.39g, 0.0025 mol) in ethanol (30 ml) containing a few drops of
concentrated hydrochloric acid is refluxed for 1 h. On cooling the title compound separated as the hydrochloride salt: yield: 0,59 g, (85%), m.p.
235-237°.
Separation of the benzamide compound and the final quinazoline alkaloid is also a bit of a hassle it seems. The condensation is performed in refluxing
ethanol, which is then supposed to precipitate the quinazoline HCl salt while holding onto residual amide.
I would assume this is due to the 2-Amino-N-(2-methylphenyl)benzamide HCl salt being more polar than the quinazoline salt due to the primary amine
group thus being more soluble in polar solvents than the quinazoline salt?
The synthesis was run using 97% ethanol which was denatured. This water may be causing problems with the pptation of the final compound too I would
think?
Another method:
A mixture of 8g isatoic anhydride and 5.5g o-toluidine in toluene (500-750 ml) is refluxed for two hours. Acetylacetone (2.5g) containing a few ml of
concentrated hydrochloric acid (to form the hydrochloride) is then added, and refluxing is continued for another hour. Evaporation of the solvent
gives the quinazoline HCl, which is purified by recrystallization from hot methanol. The yield is 80%.
Large solvent volume however, and o-toluidine not easily removed as the two step method, as it is a single pot procedure.
[Edited on 25-9-2014 by Templar]
[Edited on 25-9-2014 by Templar]
[Edited on 25-9-2014 by Templar]
He who fights with monsters should be careful lest he thereby become a monster. And if thou gaze long into an abyss, the abyss will also gaze into
thee.
|
|
|
Templar
Hazard to Self
 
Posts: 82
Registered: 17-8-2014
Location: The Sprawl, Titan
Member Is Offline
Mood: No Mood
|
|
one of the mods was helpful enough to find the reference for me.
http://www.sciencemadness.org/scipics/s-1977-24371.pdf
My guess that the amide formed , 2-Amino-N-(2-methylphenyl)benzamide, was correct.
For the first step, the amide is re-X from hexane/DCM but no ratio is given.
I think it would be most sensible to use dry ethanol for the second step, devoid of any colourings or denaturing agents (distilled)
He who fights with monsters should be careful lest he thereby become a monster. And if thou gaze long into an abyss, the abyss will also gaze into
thee.
|
|
|
Templar
Hazard to Self
 
Posts: 82
Registered: 17-8-2014
Location: The Sprawl, Titan
Member Is Offline
Mood: No Mood
|
|
Necro time.
I finally got ahold of some isatoic anhydride from a european supplier. It is 96% pure.
In 250ml xylene, 16g of isatoic anhydride and 11g of p-toluidine were combined. Yes, I did not use the ortho isomer here as I wanted to try with
something cheap before using my methoxy nitro aniline.
The mix was heated to reflux temp and left for 4 hours (the wall timer wasn't set up correctly so it went for twice as long as it should have)
The result was a dark malty transparent xylene and a fine dusty suspension in the xylene.
To this was added 5g acetylacetone and 2g conc HCl. I realized the HCl would be degrading the acetylacetone, so after those two were added, I added
another 3g acetylacetone to the reaction mix and another 1g HCl. I have left it for another hour.
Once the acetylacetone and HCl were added, the dusty tan suspension in the xylene went away, instead being replaced with a melted, almost wax like
grey material. After one hour, there is now no free suspension left floating around.
So Im going to evap off part of the solvent, and run a re-X and a MP test on the waxy material. Hopefully it is just freebase quinazolinone, because
if it is the HCl salt it will be melting around 200C, and that isnt possible in xylene.
-----------
A second synthesis was run. This time I used toluene. I hadnt wanted to get out the big drum of it and pour it out because it goes everywhere, but
whatever.
So the experiment was repeated with the same amounts of reactants, but swapping xylene for toluene. This made a big difference to the colour at the
end of the amine/anhydride condensation. The colour didnt change much after 2hrs reflux, unlike with xylene where it very clearly went a darker brown.
Obviously the 20-30C difference in reflux temp made a large difference in the amount of decomposed products.
So then 5g of acetylacetone was added. Then 3ml of conc HCl was added.
This was a mistake.
After only adding a few droplets, the half filled erlenmeyer spewed forth about 100ml of the reaction mix which overflowed everywhere. Dammit
But half is still left, so that will be continued to reflux for 1hr.
-----------------
Interesting development, the brown/black encrusted ppt that collected in the beaker with the first run using xylene has been partially dissolved in
acetone. I added an extra ml or two of conc HCl and I currently have it boiling on the hotplate. There is a light tan ppt that is clearly insoluble at
the bottom.
Isatoic anhydride and the amide that forms when it reacts with the subs. aniline are both soluble in acetone... so we shall take this out after the
acetone has cooled and do a MP test.
Hopefully it isn't some bullshit like anthranilic acid from decomposed isatoic anhydride.
------------
(3/06/2015)
So the remaining reaction mix was refluxed with the acetylacetone and 3ml conc HCl for 2hrs.
The stir bar formed a ring in the flask of a tan precipitate. It was somewhat solid upon cooling.
Currently the solid is under 200ml of 3% HCl and Im hoping it will dissolve.
I will then add 1% NaOH if it all dissolves. If this is indeed a quanzolinone, it should precipitate out as a wax.
[Edited on 2-7-2015 by Templar]
Attachment: 20150702_180253.zip (4.4MB)
This file has been downloaded 619 times
[Edited on 2-7-2015 by Templar]
[Edited on 2-7-2015 by Templar]
He who fights with monsters should be careful lest he thereby become a monster. And if thou gaze long into an abyss, the abyss will also gaze into
thee.
|
|
|
Templar
Hazard to Self
 
Posts: 82
Registered: 17-8-2014
Location: The Sprawl, Titan
Member Is Offline
Mood: No Mood
|
|
Unfortunately this synthesis does not appear to work in the manner I have implemented.
I was hoping that the solvent volumes of 200ml per 1.6g of isatoic anhydride was simply a huge excess. Maybe not.
I have two more plans to implement. Plan one would be increasing the solvent volume, or maybe trying with DMF (not very accessible though..) to form
the amide. I will have to see the solubility of isatoic anhydride in various solvents.
Plan two would involve simply heating the isatoic anhydride with the aniline dry, and then purifying and doing the ring closure using acetyl acetone
in a second stage.
Maybe even a mixture of DMF and toluene would improve solubility? If it is indeed required for this reaction that the reactants dissolve into the
solvent.
------------
So I was testing solubility of isatoic anhydride in toluene. It maxed out around 0.8g isatoic anhydride per 100ml boiling toluene.
Then I added 10ml of DMF to the mixture. Immediately the isatoic anhydride solubility rocketed up. Currently its at about 2.5g per 110ml reaction
solvent. I am going to keep pushing it and see how high it gets.
The question is... would the isatoic anhydride react with the dimethylformamide, OR would the DMF degrade into dimethylamine and would that then
react??
-----
~9% DMF in toluene solvated 5g of isatoic anhydride in 110ml solvent but I think it could do 7 or even 10g. Excellent! If it doesnt react, hopefully!!
[Edited on 3-7-2015 by Templar]
He who fights with monsters should be careful lest he thereby become a monster. And if thou gaze long into an abyss, the abyss will also gaze into
thee.
|
|
|
Templar
Hazard to Self
 
Posts: 82
Registered: 17-8-2014
Location: The Sprawl, Titan
Member Is Offline
Mood: No Mood
|
|
Results..
So a condensation in 120ml 10% DMF/toluene mix of 16g isatoic anhydride and 11g toluidine involving 2hrs reflux was then hit with acetylacetone and
~3ml conc HCl (added SLOWLY)
The mix was left to cool, and the product filtered and then dissolved in 1% HCl by gradual heating to boil.
This was then neutralized with 3% NaOH when COOL to yield 3.7g of a golden coloured tar. The tar exhibited very interesting properties, becoming soft
and gooey above 40C and being hard and brittle below 10C.
I will have to try and purify it further by acidification/basification in water.
So it does seem one can reduce solvent volume in this condensation by simply adding a small amount of DMF.
DMF did not seem to provide a lot of issues with decomposition. Acetylacetone shouldnt acetylate single amine groups... I think... its not acetic
anhydride after all.
-----
Solventless condensations of isatoic/toluidine seemed to work ok. The tar was taken up in acetone and hexane added before placing in the freezer,
resulting in a brown ppt.
The brown ppt was filtered and dissolved in 3% HCl and then basified, but this did not work well. A small amount of tan tar formed, with the majority
of the ppt a fine tan powder.
I did not think this worked so well. I will be re-attempting this to try and get more quinazolinone. I cannot find literary values for the compound I
am trying to make.
----
It is VERY difficult to get the HCl salt to ppt out of organic solvents. Sometimes it can take several cooling and warming cycles in and out of the
freezer to have something precipitate, or sometimes nothing precipitates at all.
I am unsure what kind of heat is required for formation of the HCl salt from the freebased quinazolinone. Maybe someone has some suggestions? Would a
reflux in acetone with 3% HCl likely be hot enough for the salt formation??
[Edited on 4-7-2015 by Templar]
I'll keep posting til you like it
[Edited on 4-7-2015 by Templar]
He who fights with monsters should be careful lest he thereby become a monster. And if thou gaze long into an abyss, the abyss will also gaze into
thee.
|
|
|
Templar
Hazard to Self
 
Posts: 82
Registered: 17-8-2014
Location: The Sprawl, Titan
Member Is Offline
Mood: No Mood
|
|
I have noticed that the freebase of the quinazolinone formed from the isatoic anhydride/acetylacetone route is like plasticine!
Its not the toluidine doing this, its the acetylacetone! Toluidine usually has a small degree of solubility in water, a few grams per liter.
Acetylacetone doesnt.
I noticed this when I was kneading quinazolinone freebase made from n acetyl anthranilic acid and a substituted aniline vs quinazolinone made using
the same aniline but using isatoic anhydride and acetylacetone.
When made from n acetyl anthranilic acid, the base of the quinazolinone will rapidly become crumbly when kneaded under cold water. This is because the
toluidine dissolves and washes away in the water stream, reducing plasticity.
This did not happen when the quinazolinone was made using acetylacetone. Very interesting.
I'd like to add that toluidines, specifically o-toluidine (the one I found toxicity data for) have workplace exposure limits of between 0.9 to 8
mg/m^3. Assuming a worker breathes 40m^3 in a daily shift, he will be exposed to anywhere between 14 and 40mg of toluidine.
I was often troubled about the substituted aniline impurity levels in the final quinazolinone product, but with this limit, it seems having toluidine
impurities may not be as bad as I thought.
Never the less, I will be purifying this quinazolinone further by dissolution in acidic water, followed by basification and collection of the ppt.
-----------------------
Another reaction was run using n acetyl anthranilic acid and a chosen substituted aniline.
A mix of 20g acetylated anthranilic acid and 20g substituted aniline were combined in a 100ml erlenmeyer flask. The mixture was brought up to 190C
gradually and held for 4hrs. The reaction temp fluctuated between 180 and 200C, but I noticed much more evolution of vapours at 200C than at 180.
The mixture was then poured hot into 150ml acetone (do not do this, let it cool first. I am unsure if it will react with the acetone, but its probably
best to let it cool.)
Initially I only added 6ml conc HCl. Nothing was precipitating. This is simply not enough HCl. I ran calculations and approximated I needed another
14ml more.
The acetone/HCl/quinazolinone mix was brought to the boil but only stayed liquid for about a minute after boiling as the quinazolinone salt
precipitated out.
This precipitate was filtered, and then dissolved in 3% HCl. This was then basified, the fine powdery quinazolinone freebase precipitated out, was
filtered and washed and is currently undergoing drying.
Notice how the quinazolinone base from this reaction precipitated out as a fine powder, whereas the reaction involving acetylacetone made a putty-like
base.
[Edited on 5-7-2015 by Templar]
He who fights with monsters should be careful lest he thereby become a monster. And if thou gaze long into an abyss, the abyss will also gaze into
thee.
|
|
|
applesauce
Harmless

Posts: 1
Registered: 9-1-2019
Member Is Offline
|
|
I know this thread is super old, but do you have any updates?
|
|
|
clearly_not_atara
International Hazard
    
Posts: 2692
Registered: 3-11-2013
Member Is Offline
Mood: Big
|
|
It looks like the original question resulted from a mistakenly omitted "NH2" in a Kekule diagram. I don't think there's much to update unless you want
more experimental details about how to make this particular controlled substance, but you're not allowed to ask for that, of course.
[Edited on 04-20-1969 by clearly_not_atara]
|
|
|
|