madscientist
National Hazard
   
Posts: 962
Registered: 19-5-2002
Location: American Midwest
Member Is Offline
Mood: pyrophoric
|
|
Picramic acid synthesis
I have no detailed information on preparing picramic acid. Does anyone have information to share? The following is the information that I have:
picramic acid (picraminic acid; 2-amino-4,6-dinitrophenol; dinitroaminophenol) C6H2(NO2)2(NH2)OH
Derivation: By partial reduction of picric acid.
I weep at the sight of flaming acetic anhydride.
|
|
|
Nick
Harmless

Posts: 11
Registered: 25-5-2002
Location: England.
Member Is Offline
Mood: No Mood
|
|
"In a beaker place 90 ml warm water and 1.5 grams of
sodium hydroxide. Mix these with a glass rod until all the NaOH has
dissolved. Dissolve 9 grams of picric acid crystals in the NaOH-water
solution by stirring. Label this beaker solution #1. In a 500 ml beaker 3 ml
of water is placed. Dissolve 7.5 grams of sulfur and 7.5 grams of sodium
hydroxide by stirring the solution. Boil this solution over a heat source.
When the solution turns dark red remove and allow the liquid to cool. Label
this solution #2. Add this cooled solution #2 in three portions, to
solution #1. Stir with a glass rod while the liquid is being added. Again
allow the solution mixture cool. Filter this mixture through filter papers
(coffee filter, paper towels). Small red particles will gather on the
paper. Discard the liquid. Dissolve these red particles in 180 ml of boiling
water. Remove and filter this hot liquid through a filter paper (coffee
filter, paper towels). Discard the particles left on the paper and label
the liquid left #3. To Solution #3 with an eyedropper slowly add sulfuric
acid (Janitor supply, boiled battery acid) to the filtered solution until
it turns orange brown. Add an additional 7.5 grams of acid to the liquid."
(Taken from KIBC)
This will form it in solution, just boil it down a bit and chill it to get picramic acid crystals.
|
|
|
PrimoPyro
on fire
  
Posts: 122
Registered: 7-8-2002
Member Is Offline
Mood: No Mood
|
|
Its a partial reduction where only one nitro function is reduced to an amine. Or were you asking something else??
PrimoPyro
|
|
|
kingspaz
Hazard to Self
 
Posts: 55
Registered: 23-7-2002
Location: UK
Member Is Offline
Mood: No Mood
|
|
i think hes looking for info 
R-NO2 + Na2S + H2O ---> R-NH2 + Na2SO3
i know its Na2Sx  ...but you get the idea ...but you get the idea
i have no idea why only the nitro group on carbon 2 is reduced...but wait...thats the same as reduction on carbon 6 if you flip the molecule. so it
must be reduced on carbon 2 or 6 then because NH2 is an electron adding species (i think) then that prevents further reduction somehow....
|
|
|
Megamarko94
Hazard to Self
 
Posts: 68
Registered: 31-12-2010
Member Is Offline
Mood: No Mood
|
|
at what conc. is sulfuric acid can 96% be used or it must be diluted.
|
|
|
g3ovn
Harmless

Posts: 12
Registered: 8-11-2010
Member Is Offline
Mood: No Mood
|
|
D'oh!
I didn't know it was a 2002 thread!
------------------
There are synthesis info in the library, eg. Dyes classified by intermediates p.454 "FORMATION.—From picric acid by reduction,
using sodium hydrogen sulfide or sodium sulfide".
Fundamental Processes of Dye Chemistry p.152
In a glass or iron container of at least 2.5-liter capacity, a solution
of 10 grams of picric acid and 10 grams of 35 per cent sodium hydroxide
in 600 cc. water is heated to 55°C, and to this is added, with vigorous
stirring over a period of 10 minutes, a solution of crystalline sodium
sulfide in 100 cc. water. When this addition is completed, an additional
127.5 grams of pulverized picric acid is added in teaspoon portions, and
simultaneously a solution of 220 grams of sodium sulfide in 400 cc. water
is introduced, the additions of the two reagents being completed at the
same time (within about 10 minutes in all). If the temperature goes
above 65°, ice is added. Stirring is continued for 10 minutes more, then
the mixture is poured onto 400 grams of ice, precipitating the sodium
picramate completely. After 10 hours, the mixture is filtered, and the
precipitate is washed with 10 per cent salt solution. Free picramic acid
is obtained by dissolving the sodium salt in 500 cc. water, warming the
solution to 80°, and acidifying, with stirring, with dilute sulfuric acid.
The mixture, which should be just acid to Congo red, is allowed to cool
and stand for 10 hours. The product is then filtered off, yielding about
100 grams of pure material.
Variation. The partial reduction of picric acid can be effected in
various ways. Instead of adding the picric acid gradually, and thus using
it to neutralize the alkali formed:
4 X—NO2 + 6 Na2S + 7 H20 -> 4 X—NH2 + 6 NaOH + 3 Na2S203
(see also page 114) the sodium salt can be reduced and the necessary
amount of hydrochloric acid added simultaneously.
For example, 0.6 mole (137.5 grams) of picric acid is mixed with
1.2 liters of water and 36 grams of soda ash at 50°. Solution is not complete.
When the carbon dioxide has been expelled, a solution of 1 mole
(240 grams) of crystalline sodium sulfide in 450 cc. water is added, with
good stirring, during the course of 30 minutes. Simultaneously, a mixture
of 108 grams of 30 per cent hydrochloric acid and 300 cc. water is
added at such a rate that this addition requires about 1 minute longer
than that of the sodium sulfide solution. Stirring is then continued, with
out heating, for 30 minutes, and the mixture is filtered after 12 hours.
The precipitate is washed with 100 cc. saturated salt solution. This
crude sodium picramate is dissolved in 2 liters of water, and the solution
is filtered and poured into a hot (90°) solution of 70 cc. 30 per cent
hydrochloric acid in 400 cc. water. The pure picramic acid is precipitated
completely after 24 hours, and is filtered off, washed with a small
amount of water, and dried at 80°. The yield is about 100 grams, or 83
per cent of the theoretical amount.
-------------------------------------------------------------------------------------------------------------
Zong-Wei Yanga, et al "Synthesis and Characterization of spherical 2-diazo-4,6-dinitrophenol" Journal of Hazardous Materials 177
(2010) 938–943
Synthesis of sodium picramate: 400 kg of water and 45 kg of
picric acid (containing 20% water) were added to an 800 L steel neutralization
reactor fitted with a stirrer, a thermometer and water
condenser, then the suspension was stirred. After mixture was
heated up to 60 ◦C, about 50–55 kg of 12.7% sodium sulfide solution
was added to the suspension of picric acid by constant flow pump.
During the addition, the temperature of reaction mixturewasmaintained
at 60 ◦C. When the color of reaction solution changed from
yellow to red and pH value of reaction solution was 8–9, addition
was then stopped. The reaction mixture was filtered to remove foreign
material such as sulfur, and the dark red solution of sodium
picrate was obtained.
The solution of sodium picrate was added to reduction reactor,
and agitator was started. The solution of sodium picrate was
warmed up to 50 ◦C. 160–165 kg of 12.7% sodium sulfide solution
was added with multiholes by constant flow pump within 35 min.
The temperature was kept at about 50–55 ◦C during the reduction.
After completion of addition of the sodium sulfide solution, agitation
was continued for another 5min at the same temperature. The
reaction mixture was cooled to room temperature and filtered, and
the precipitate was washed with water to get jujube red color and
wedgeshaped crystals of sodium picramate, in a yield of about 36 kg
(dry basis). Finally, the basic mother liquor was equally divided into
two portions, which can be used as a partial reaction liquid of next
two batches for synthesizing sodium picramate again, respectively.
----------------------------
I also uploaded an interesting article intitled "The Alkaline Reduction of Aromatic Nitro Compounds with Glucose", which says that a guy in 1895 made
it reducing the nitrogroup with an alkaline glucose solution.
-----------------------------
You should had asked it in the energetic materials, people there like DDNP.
Attachment: The Alkaline Reduction of Aromatic Nitro Compounds with Glucose.pdf (286kB)
This file has been downloaded 1264 times
[Edited on 11-7-2011 by g3ovn]
[Edited on 11-7-2011 by g3ovn]
|
|
|
Bot0nist
International Hazard
    
Posts: 1559
Registered: 15-2-2011
Location: Right behind you.
Member Is Offline
Mood: Streching my cotyledons.
|
|
DDNP
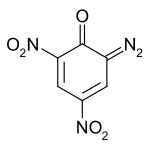
Picramic acid

Are they the same? Forgive me if this is a stupid remark.
[Edited on 12-7-2011 by Bot0nist]
U.T.F.S.E. and learn the joys of autodidacticism!
Don't judge each day only by the harvest you reap, but also by the seeds you sow.
|
|
|
g3ovn
Harmless

Posts: 12
Registered: 8-11-2010
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Bot0nist  |
DDNP

Picramic acid

Are they the same? Forgive me is this is a stupid remark.
[Edited on 12-7-2011 by Bot0nist] |
I meant that they know well how to synthetize the picramic acid to make DDNP. Im aware they are different compounds. By not knowing the synthesis was
in a book about dyes i assume it was going to be used to make DDNP. That's why i said that.
|
|
|
Bot0nist
International Hazard
    
Posts: 1559
Registered: 15-2-2011
Location: Right behind you.
Member Is Offline
Mood: Streching my cotyledons.
|
|
I couldn't find much on the use of picramic acid to diazodinitrophenol in the forum. Maybe you should start a thread on it. I did not mean my post as
a jab. I really just didn't know of its use. I found a few things concerning DDNP, though I only scanned through some of the material.
<a href="http://www.sciencemadness.org/talk/viewthread.php?tid=433&page=2#pid65652">In the picramic acid from TNP thread</a>
also
<a href="http://www.sciencemadness.org/talk/files.php?pid=65652&aid=1376">a DDNP,pdf</a>
<a href="http://www.sciencemadness.org/talk/viewthread.php?tid=152#pid920">From a primaries thread,</a> which has several good pdf on the
subject.
[Edited on 12-7-2011 by Bot0nist]
U.T.F.S.E. and learn the joys of autodidacticism!
Don't judge each day only by the harvest you reap, but also by the seeds you sow.
|
|
|