zbde00
Harmless

Posts: 25
Registered: 18-2-2005
Member Is Offline
Mood: No Mood
|
|
how to remove the Br atom?
Is there any way to remove the Br atom except for Pa/C reduction?
thanks.
please see the picture
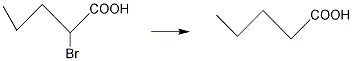
|
|
|
sparkgap
International Hazard
    
Posts: 1234
Registered: 16-1-2005
Location: not where you think
Member Is Offline
Mood: chaotropic
|
|
I wouldn't use protactinium to reduce my alpha-halo acids. 
Of course, I know you meant to want to avoid Pd on C.
Anyway, looking at it, the best I can think of is to use a hydride reducing agent that will leave the carboxylic function unscathed. Sodium
borohydride, for instance.
Anyone have better ideas?
sparky (^_^)
"What's UTFSE? I keep hearing about it, but I can't be arsed to search for the answer..."
|
|
|
I am a fish
undersea enforcer
   
Posts: 600
Registered: 16-1-2003
Location: Bath, United Kingdom
Member Is Offline
Mood: Ichthyoidal
|
|
If you have access to potassium cyanide, you could:
1. Heat the 2-bromopentanoic acid strongly, decarboxylating it into 1-bromobutane.
2. Heat (under reflux) the 1-bromobutane with an ethanolic solution of potassium cyanide, converting it into pentanenitrile.
3. Heat (under reflux) the pentanenitrile with dilute hydrochloric acid, converting it into pentanoic acid.
1f `/0u (4|\\| |234d 7|-|15, `/0u |234||`/ |\\|33d 70 937 0u7 /\\/\\0|23.
|
|
|
sparkgap
International Hazard
    
Posts: 1234
Registered: 16-1-2005
Location: not where you think
Member Is Offline
Mood: chaotropic
|
|
Little bone to pick: your suggestion is kind of longwinded. Three steps? 
Besides, only beta-keto acids undergo reliable thermal decarboxylations. alpha-halo acids like 2-bromopentanoic acid wouldn't give satisfactory
amounts of corresponding halo alkanes.
sparky (^_^)
"What's UTFSE? I keep hearing about it, but I can't be arsed to search for the answer..."
|
|
|
Marvin
National Hazard
   
Posts: 995
Registered: 13-10-2002
Member Is Offline
Mood: No Mood
|
|
Sodium, t butyl alcohol in THF maybe?
I'm wondering about zinc amalgam, or even just zinc in dilute acid but a quick check doesnt find anything convincing that the usual oxygen or
sulphur alpha to a carbonyl method will work. I wonder also if adding a little iodide would help catalyse the thing, -I will replace with -H at the
drop of a hat and it should substitute for Br.
|
|
|
BromicAcid
International Hazard
    
Posts: 3227
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
There is a name reaction, whose name escapes me (along with its exact function and conditions under which it occurs  ) that removes bromine atoms from organic substituents, involving the use of silver
oxide. Not much to go on with no guaranteed useful information at the end but maybe you should investigate further. ) that removes bromine atoms from organic substituents, involving the use of silver
oxide. Not much to go on with no guaranteed useful information at the end but maybe you should investigate further.
|
|
|
Marvin
National Hazard
   
Posts: 995
Registered: 13-10-2002
Member Is Offline
Mood: No Mood
|
|
Odd reagent to use, you arn't thinking of the Walden inversion are you? Moist silver oxide to replace with -OH. Silver oxide is also a mild
oxidising agent, when what is being done is a reduction. Though formation of AgBr does drive a lot of odd reactions forward.
|
|
|
Kinetic
Harmless

Posts: 45
Registered: 31-12-2004
Member Is Offline
Mood: fine
|
|
Removing organic halides
The first idea which came to me was refluxing the acid in concentrated HI, a method that has been known for over a century to reduce alpha-haloketones
to ketones. I don't know whether it can reduce alpha-bromo acids though.
A quick literature search suggests there are not too many practical procedures for the dehalogenation of alpha-bromo acids, though much of what I
found appears to agree with what Marvin has said above. Sodium amalgam can indeed effect the transformation; if you have access to the ancient
scientific literature, see Justus Liebigs Ann. Chem., 130, 18 (1864) for a procedure. This article was actually written by
Kekule.
Dissolving metals are probably your best bet. There is a review on the dehalogenation/hydrogenolysis of organic halides in Synthesis,
1980, 425-452 which you may like to take a look at. Also, in Synlett, 2001, 485-488, Raney-Ni is used to
dehalogenate chloroacetic acid to acetic acid in 2 hours, giving 90% yield. The representative procedure is as follows (this works for a large number
of organic halides):
Typical experimental procedure
1.5 g of a slurry of commercial aqueous Raney nickel (Fluka, cat.no. 83440)* was added to a solution of halide (150 mg) in THF (10 mL) and the mixture
was further vigorously stirred at room temperature or under reflux (except for chlorides, which were refluxed in the presence of double amount of
Raney nickel) for the especified time. The mixture was diluted with ether and filtered through silica gel, and the solvent was evaporated to yield the
reduced compound.
* The Raney nickel was weighed as an aqueous slurry after removing four fifth of water.
Zinc in acetic acid has also been used for the hydrogenolysis of organic halides.
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
The compound concerned is alpha (or 2-)-bromo-valeric acid, or alpha-bromo-pentanoic acid, in which it is desired to replace the Br with H. It would
probably have to be esterified first, to protect the carboxylic acid group, e.g. by reaction with diazomethane (quick but poisonous and explosive) or
by reaction with an alcohol with a small amount of a mineral acid.
Once this is done, a possibility would be reaction with an electropositive metal, which would result in the metal bromide as an exothermically-favored
byproduct The debrominated ester could be hydrolysed back to the carboxylic acid, which could be separated from the alcohol by distillation (provided
there is no constant-boiling azeotrope between them).
|
|
|
zbde00
Harmless

Posts: 25
Registered: 18-2-2005
Member Is Offline
Mood: No Mood
|
|
thank everyone.the substrate in the first picture is comming from the below picuture.
If everyone have ideas about this conversition about removing amino group,i will be very delighted.
please see the attachment.

|
|
|
unionised
International Hazard
    
Posts: 5102
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
If anyone can think of a suitable way of protecting the acid then conversion to the Grignard then adding water would work.
Probably not a lot of use in this instance, but if anyone searches for "removing bromine" or debromination for some other substrate this
might help them.
|
|
|
kyanite
Hazard to Self
 
Posts: 86
Registered: 30-6-2004
Location: is not important!
Member Is Offline
Mood: lactamic
|
|
Maybe reacting the bromine acid with lithium or magnesium, then with water. Unless you are making massive amounts, then it could get expensive. Also
turn it into a salt first, or else youll be wasting metal.
|
|
|
sparkgap
International Hazard
    
Posts: 1234
Registered: 16-1-2005
Location: not where you think
Member Is Offline
Mood: chaotropic
|
|
About zbde00's second question, methinks diazotization would be the first thing to do in removing the amino group.
Marvin, Walden inversion is the observation that SN2 reactions proceed with inversion of configuration. This does not necessarily involve using silver
oxide.
sparky (^_^)
P.S. esterify/Grignard/hydrolyze looks good also.
sparky (^_^)
"What's UTFSE? I keep hearing about it, but I can't be arsed to search for the answer..."
|
|
|
Marvin
National Hazard
   
Posts: 995
Registered: 13-10-2002
Member Is Offline
Mood: No Mood
|
|
The original Walden inversion is the method of treating a chiral alcohol with PCl5 to form the chloride, and then with moist silver oxide to
regenerate it, but in the inverted configuration.
I'm sure this isnt the only method of doing it, but it was the only non oxidising use for Ag2O I knew offhand so I wondered if replacing -Br with
-H had been confused with a special way of replacing -Br (or another halogen) with -OH.
On some digging silver oxide is also used in some hydrohalogen removals, but this doesnt help either.
Several people have suggested generating an ester, but aside from being unsubtle and adding 2 extra steps... Esters arnt that well protected and the
alpha carbon should be much more easily reduced anyway. For example a sodium reduction on the ester would also cause a Claisen condensation
potentially, and an attempted Grignard/water would work if the Grignard reaction itself did not include esters, aside from potentially heavy losses
due to coupling reactions.
I think a specific reducing agent is the key to doing this one well and that means trawling archives I dont have access to.
As for removal of a -NH2 groups alpha to a carboxyl for an ester there is an SmI2/THF method specific to this problem that will come up on a search.
Diazotising may or may not succeed, as a general aliphatic method of course you get an alcohol right away. In the case it goes to an alcohol the zinc
reduction may then work for the product. If not, and the carbonyl or ester may exert enough of an influence to make the diazo group stick it has
other possibilities.
|
|
|
alchemie
Harmless

Posts: 36
Registered: 4-6-2003
Member Is Offline
Mood: variational
|
|
the halogen can be removed with Tri-n-ButylTin hydride and a radical initiator like AIBN (azo-bis-isobutyronitrile). The carboxylate group might have
to be protected, a simple ester should do.
This is a relatively common reagent and it shouldn't be difficult to find a procedure and adapt it to this substract.
|
|
|