joe_aldehyde
Hazard to Self
 
Posts: 68
Registered: 26-3-2005
Member Is Offline
Mood: what is mood?
|
|
methylenation of catechols using NaF
i know about one methylenation that uses KF [9] Tet Lett 38, 3361-3364 (1976), what about NaF? is the solubility too low to be of any use? just asking
because i have some sitting on the shelf.
i wonder if anhydrous conditions are a must. the paper mentioned above has been referenced several times on rhodium.
|
|
|
daeron
Hazard to Self
 
Posts: 74
Registered: 26-3-2005
Member Is Offline
Mood: cancerogenic
|
|
NaF can be used,the yields will differ slightly.It is a common practise that you use NaX instead of KX and vice versa
Swim doesnt have the data but the solubility is simmilar too
goddamn i said i wont be returning on this forum,but something keeps calling me..
PS swim knows a professor who did a simmilar rxn using NaF with no problems
|
|
|
joe_aldehyde
Hazard to Self
 
Posts: 68
Registered: 26-3-2005
Member Is Offline
Mood: what is mood?
|
|
aha aha, let's see.
while i would favor the MW-assisted methylenation a lot, if i only had the details...
|
|
|
CherrieBaby
Hazard to Self
 
Posts: 91
Registered: 4-3-2005
Location: London
Member Is Offline
Mood: No Mood
|
|
I think the K+ acts to form the phenolate first which then reacts with CH2X2. I bet they would've tried NaF; I don't think it would work.
But, hey, try it and tell us what happened.
I would go with the reaction details I just posted if I were you (using CH2I2). 95% yield can't be sniped at. I bet a different phase transfer
catalyst would substitute for TBAB. You can make CH2I2 from CH2Br2 or CH2Cl2 by halogen swap.
There's also a methylenation using CH2Cl2, PTC and NaOH in aq. solvent with a trace of I2 or MI to act as a promoter (Japanese patent). They say
for each mole of reactant, use 0.1 mole PTC and 0.01 mole of I2/I salt. I imagine this would have to be done under pressure (maybe reflux but that
would takes a LONG time). I can't, of course, read Japanese. Which leads me to imagine that the same would go better under microwave but only in
a pressurised container (or microwave reflux setup). CH2Cl2 is just too volatile, otherwise. Can you get hold of those PTFE pressurised digestion
containers or are they too expensive? I have "microwave pressure cookers" which I got from my grocery store for making rice. I wonder what
would happen trying rxns out in those? (The polypropylene material wouldn't be a problem but they are too flimsy). Or, time to get the hacksaw
out and make that microwave reflux setup? Can anyone think of an alternative to the PTFE digestion containers for pressurised microwave rxns?
Plus there are the other methylenations done in aprotic solvent and the PTC/aq. solvent/MOH/CH2Br2.
|
|
|
joe_aldehyde
Hazard to Self
 
Posts: 68
Registered: 26-3-2005
Member Is Offline
Mood: what is mood?
|
|
i don't think that K+ has anything to to with the phenolate salt, in the original paper it said something about a "strong new bond being
formed by F---HO". no K+ anywhere 
i have CH2Br2 which i think ought to be sufficiently reactive for methylenation, yields are not very important for me right now (it could drop by some
20% and i'd still be happy). and i don't have NaI, but i do have a huge bottle of alcoholic iodine/iodide tincture, if that's any use
in the methylenation.
personally, i'm a bit anxious about trying pressurized rxns, especially using a microwave. i wouldnt try that unless i had a proper lab.
if you have reference for other methylenationes, just post away. i'd be happy to complete my collection.
thx.
j
|
|
|
daeron
Hazard to Self
 
Posts: 74
Registered: 26-3-2005
Member Is Offline
Mood: cancerogenic
|
|
im tired..
K+ does not bond!!None of the proposed mechanisms even mentions this.
if youre not interested in the yields then use CH2Br2 without fear;plus
from what ive heard on the hive,using ch2Br2 may produce even higher yields(not confirmed).
CherrieBaby:
Let say im more of a org.chem.eng than a chemist..sooo you have 2 options:atm p and long reflux;
and a p,t rxn.NOT mw/p rnx;unless you do some serious calculations dont even think about doing it.
Plus you must explore all the possibilities of voluime increasing side rxns.
....that is unless you have a specific procedure..hmm?Do mw rxns work by supeheating the mix/activating the
molecules/and even changing conformations..
And ive asked around about tbab substitutes-a..336 works wonderfully
goddamn i said i was going to sleep...
|
|
|
runlabrun
Hazard to Others
  
Posts: 172
Registered: 4-12-2004
Member Is Offline
Mood: No Mood
|
|
The use of flouride salts in this proceedure was to form strong hydrogen bonding with the catchetol alcohol groups... thereby decreasing the risk of
polymerisation reactions of the two catchetol groups and reducing the yield of the methylenedioxybenzene product.
As long as the flouride salt can be reasonably soluble in the solvent and the metal cation does not complex with the flouride ions once in solution
your fine...
NaF, KF..... why would there be a difference? KF was used probably because it was right there on the shelf.... But NaF would work fine.
-rlr
|
|
|
joe_aldehyde
Hazard to Self
 
Posts: 68
Registered: 26-3-2005
Member Is Offline
Mood: what is mood?
|
|
nice to know, while i guess that Na+ won't complex with F-, my general chemistry lectures happened a while back but i still know that 
the proposed solvent is anhydrous DMF, does anybody think that it has to be reasonably anhydrous to obtain enough product? i can't see any role
that water would play in this reaction, except for solvating the NaF (which could be avoided by using an excess of it) and forming hydrogen bonds with
the hydroxy groups (not so bad, most likely). doesn't DMF stink like hell?
|
|
|
runlabrun
Hazard to Others
  
Posts: 172
Registered: 4-12-2004
Member Is Offline
Mood: No Mood
|
|
Where did you get the idea water was required? its used in the end to remove DMF from the extracted product but not for the reaciton itself...
anhydrous DMF is the solvent used in the page on rhodium about the proceedure...
Anhydrous is a fixed term, there is no reasonably about it... its water free... i dont know what effect water would have on the reaction so i would
say yes, make certain its anhydrous...
you want NaF to be solvated.... how else would you get F- in solution to get the desired hydrogen bonds with the catchetol? the hydrogen bonding is
what prevents most of the polymerisation of the two catchetol groups...
-rlr
|
|
|
joe_aldehyde
Hazard to Self
 
Posts: 68
Registered: 26-3-2005
Member Is Offline
Mood: what is mood?
|
|
rlr, i think you got me wrong.
i didn't think that water is needed in the reaction, i just thought that i might get ahold of DMF which is NOT anhydrous, and i don't think
that i'd take the pain and dry it.
all i was musing about was that water might be causing problems during the reaction, which i cannot figure out on my own though. thus my question.
|
|
|
CherrieBaby
Hazard to Self
 
Posts: 91
Registered: 4-3-2005
Location: London
Member Is Offline
Mood: No Mood
|
|
If anhydrous DMF is specified then you better make it anhydrous before you use it.
Look up the DMF on page 192 of "The Purification of Laboratory Chemicals", 4th ed., Armarego & Perrin. [This has been scanned, you can
get it on eMule, on the various FTPs and maybe even from the web.]
Purification of Organic Chemicals, 4e, p. 192. DMF entry.
N,N-Dimethyl formamide (DMF) [68-12-2] M 73.1, b 76°/39mm, 153°/760mm, d 0.948, n(25) 1.4269. Decomposes slightly
at its normal boiling point to give small amounts of dimethylamine and carbon monoxide. The decomposition is catalysed by acidic or basic materials,
so that even at room temperature DMF is appreciably decomposed if allowed to stand for several hours with solid KOH, NaOH or CaH2. If these reagents
are used as dehydrating agents, therefore, they should not be refluxed with the DMF. Use of CaSO4, MgSO4, silica gel or Linde type 4A molecular sieves
is preferable, followed by distn under reduced pressure. This procedure is adequate for most laboratory purposes. Larger amounts of water can be
removed by azeotropic distn with benzene (10% v/v, previously dried over CaH2), at atmospheric pressure: water and benzene distil below 80°. The
liquid remaining in the distn flask is further dried by adding MgSO4 (previously ignited overnight at 300-400°) to give 25g/L. After shaking for one
day, a further quantity of MgSO4 is added, and the DMF distd at 15-20mm pressure through a 3-ft vacuum-jacketed column packed with steel helices.
However, MgSO4 is an inefficient drying agent, leaving about 0.01M water in the final DMF. More efficient drying (to around 0.001-0.007M water) is
achieved by standing with powdered BaO, followed by decanting before distn, with alumina powder (50g/L; previously heated overnight to 500-600°), and
distilling from more of the alumina; or by refluxing at 120-140° for 24h with triphenylchlorosilane (5-10g/L), then distilling at ca 5mm pressure
[Thomas and Rochow JACS 79, 1843, 1957]. Free amine in DMF can be detected by colour reaction with 1-fluoro-2,4-dinitrobenzene. It has also been
purified by drying overnight over KOH pellets and then distd from BaO through a 10 cm Vigreux column [Experimental Cell Research 100, 213, 1976]. [For
efficiency of desiccants in drying dimethyl formamide see Burfield and Smithers [JOC 43, 3966, 1978, and for a review on purification, tests of purity
and physical properties, see Juillard. PAC 49, 885, 1977].
It has been purified by distilling from K2CO3 under high vac and fractionated in an all-glass apparatus. The middle fraction is collected, degassed
(seven or eight freeze-thaw cycles) and redistd under as high a vacuum as possible [Mohammad and Kosower JACS 93, 2713, 1977].
[Edited on 1-4-2005 by CherrieBaby]
|
|
|
Sandmeyer
National Hazard
   
Posts: 784
Registered: 9-1-2005
Location: Internet
Member Is Offline
Mood: abbastanza bene
|
|
| Quote: | Originally posted by CherrieBaby
I think the K+ acts to form the phenolate first which then reacts with CH2X2. |
Potassium ion is not even basic, so this is not what happens..
[Edited on 29-5-2005 by Sandmeyer]
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
I was reading reference 9 (JACS 99, 498 (1977)) of the above Tet. Lett. ref. (same authors), where they use KF and CsF again, (and RbF) and noticed that they tried LiF and NaF on not-catechol and got little
to no yield. Now this does not say what happens to phenols, but they do seem very prejudiced against NaF for their work. There is certainly a
correlation shown between reactivity and row on the periodic table, with phthalimide. You'd think that if the determining factor was
solubility-related, they would say so; they don't. In any case KF is little more expensive to make than NaF for the hobbyist.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
It is a quite simple case of solvation issue actually. You have an alkali fluoride used as a base. In order for it to behave like a good base it needs
to fulfill two conditions:
1.) The fluoride ion must be unsolvated! This means only aprotic solvents can be used since F<sup>-</sup> is an excellent H-bond acceptor.
Fluoride anion, unlike hydroxide, is only a strong base when in aprotic solvents, while in water it is just as a mediocre base as an acetate or
bicarbonate ion is.
2.) The cation counterion must be of such nature as to be preferentially solvated by the solvent rather than liganding with F<sup>-</sup>.
For similar reasons as described above for fluoride basicity, the small unpolarizable cations are actually acids in aprotic, particularly nonpolar
media (for example, LiClO4 in diethyl ether is a strong acid!). The smaller the cation the more it will be acidic and will hang to any dipole or anion
it can found.
Therefore, due to reason (2) it is necessary to use a very polar solvent to solvate all the alkali metal cations and due to reason (1) an aprotic one
in order not to solvate any fluoride anions. Hence solvents like DMF, DMSO, NMP, etc.
Due to reason (2) the basicity in such solvents will in any case follow this line: LiF < NaF < KF < RbF < CsF.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
So perhaps NMe4F would work even better than CsF?
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
I'm not sure, but I think the Me<sub>4</sub>N<sup>+</sup> cation behaves similarly as Na<sup>+</sup> in regard to
solvation properties. Higher fluoride quats like Bu<sub>4</sub>NF are nearly impossible to dry from the hydration water without
decomposing them. Obviously, in regard to polarizability, the tetraalkylphosphonium or trimethylsulfonium fluorides would be even better, but as far
as I know such fluorides would be even much less thermally stabile.
Not that these alternatives have any practical meaning… potassium fluoride simply must be used for any such alkylation, or else the method is just
an academic curiosity without any applicability. If it would be about efficiency, chemists would use
Cs<sub>2</sub>CO<sub>3</sub> any time over K<sub>2</sub>CO<sub>3</sub> as a base for alkylations, but
just compare the price of the two and you see why they rarely do.
EDIT: I don't want to make free publicity, but I found that one of our favorite chemical suppliers actually made a nice review on cesium salts as
bases in organic chemistry which explains the issue of Li-Na-K-Rb-Cs cations and their solvation: CATALYSTS CESIUM
Excerpt...
[Edited on 5/2/2008 by Nicodem]
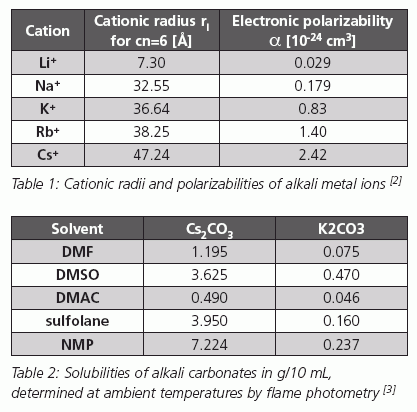
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|