| Pages:
1
2 |
The WiZard is In
International Hazard
    
Posts: 1617
Registered: 3-4-2010
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by hissingnoise  | Ozone is an interesting oxidiser, but its synthesis is difficult and inefficient; pity it can't be formed by a contact process like the catalytic
oxidation of so2 on pt. gauze.
If this gas could be produced simply and cheaply it would be a boon to headcases like us. Our favourite acids(hno3-h2so4)could be easily made, with a
minimum of reagents and glassware. |
Apparently the most chemical efficient way of producing ozone
it that discovered by Moissan (1891) dropping water into fluorine.
"At 0o , oxygen containing up to 21 per cent of ozone by weight
was obtained". Mellor V1 p 887.
Mellor redux — Ruff & Tschrich obtained ozonized oxygen by
the action of osmium octafluroide on soda lye.
(All you have to do is separate da octa from da hexa and tetra...
F's....! See Mellor V15 p714.)
A chemical engineering note :—
This from K&O 2nd 14:421
Best reported results
Energy yield g/kWh ---- Conc.
electric discharge in O2 --- up to 150 --------- up to 6 vol%
electrolysis of water --- up to 12 --------- 20
photochemical
1850-2537 A's --- up to 25 --------- 0.25
1400-1700 A's --- do --------- do
radiochemical
using O2 gas --- 220 ? --------- 60 ppm
using liquid O2 --- 108 ---------- 5 mole %
thermal --- 56 -------- 0.33 mole %
Ullmann sez corona discharge in the most common commercial
method of production.
djh
----
Like the comic book undetectable
ice dart, I have often though that
liquid ozone would make the ultimate
no trace explosive.
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
| Quote: |
Like the comic book undetectable
ice dart, I have often though that
liquid ozone would make the ultimate
no trace explosive. |
File under "Unsolved Cases of Spontaneous Explosive Dismemberment" . . .
|
|
|
Aqua_Fortis_100%
Hazard to Others
  
Posts: 302
Registered: 24-12-2006
Location: Brazil
Member Is Offline
Mood: †
|
|
There is a lot of good info on ozone generation..
http://www.valdosta.edu/~tmanning/research/ozone/intro.html
http://www.valdosta.edu/~tmanning/research/ozone/
http://www.lenntech.com/library/ozone/reaction/ozone-reactio...
Handbook of Ozone Technology and Applications, vol 1. – Rip G. Rice/Aharon Netzer – Ann Arbor Science
and more...
DIY (Bigclive page):
http://www.bigclive.com/ozone.htm
http://www.bigclive.com/oz.htm
My home generator was based mostly on OTC and discarded things..
Ive used an aquarium pump, an old flyback tranformer (non-rectified), PC cooler, a dead CFL circuit (and its glass tubes), and a PC PSU...



Actually, Ive used another dead CFL (of a lower wattage rating):

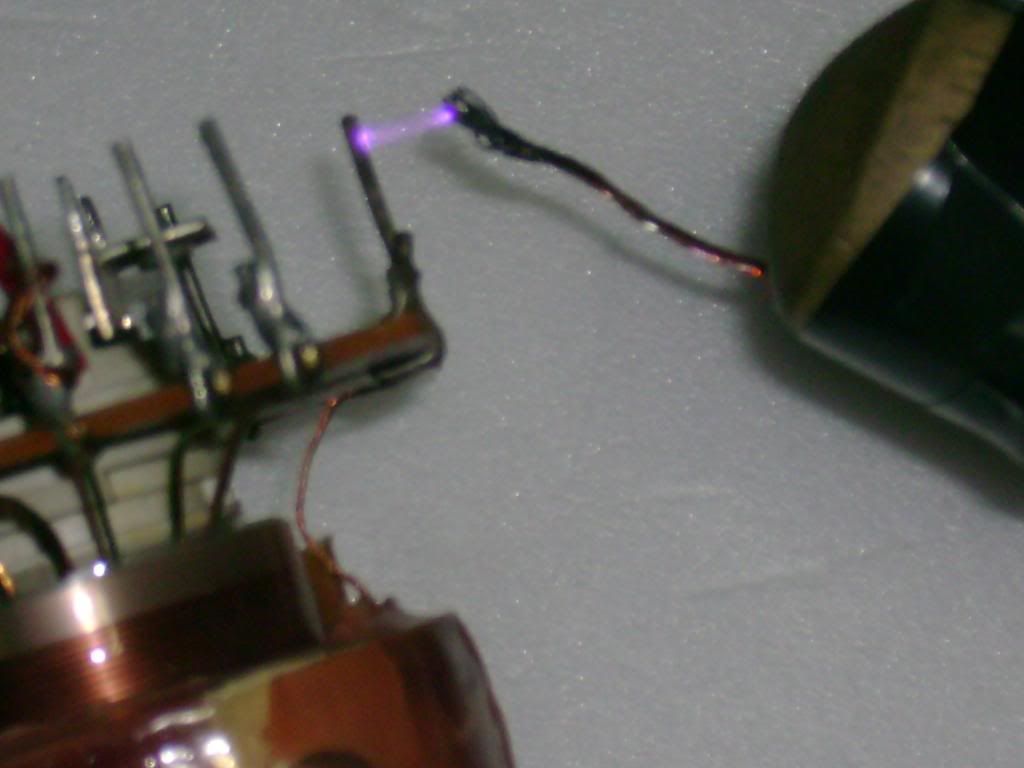
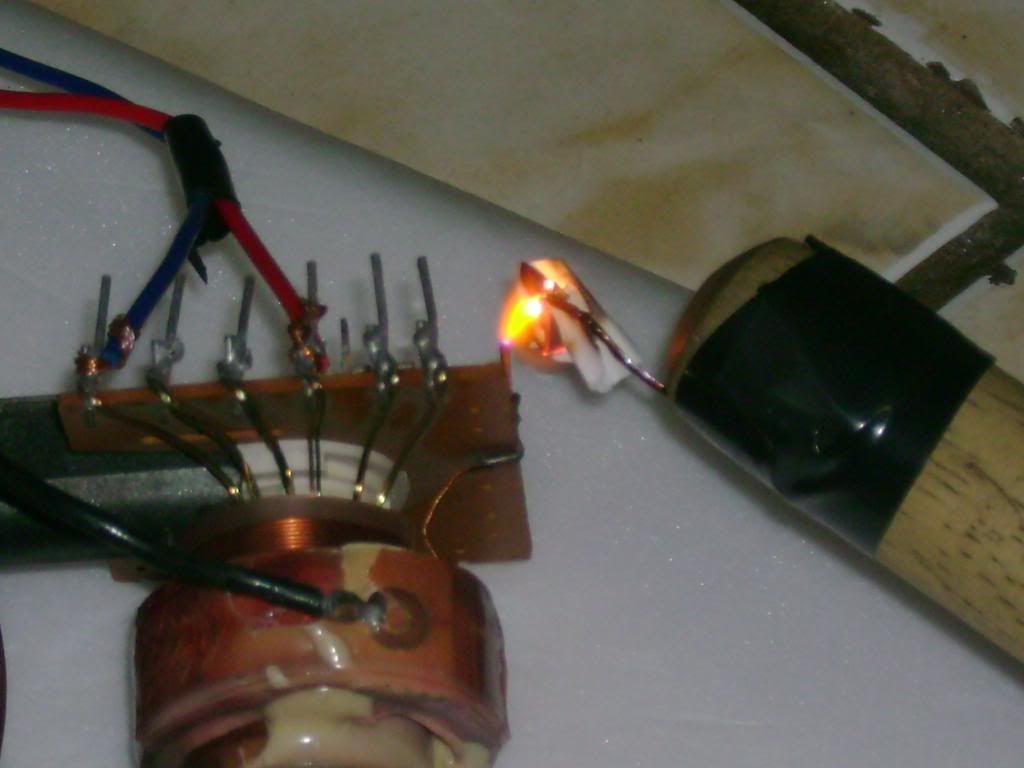
The actual "ozonizer":


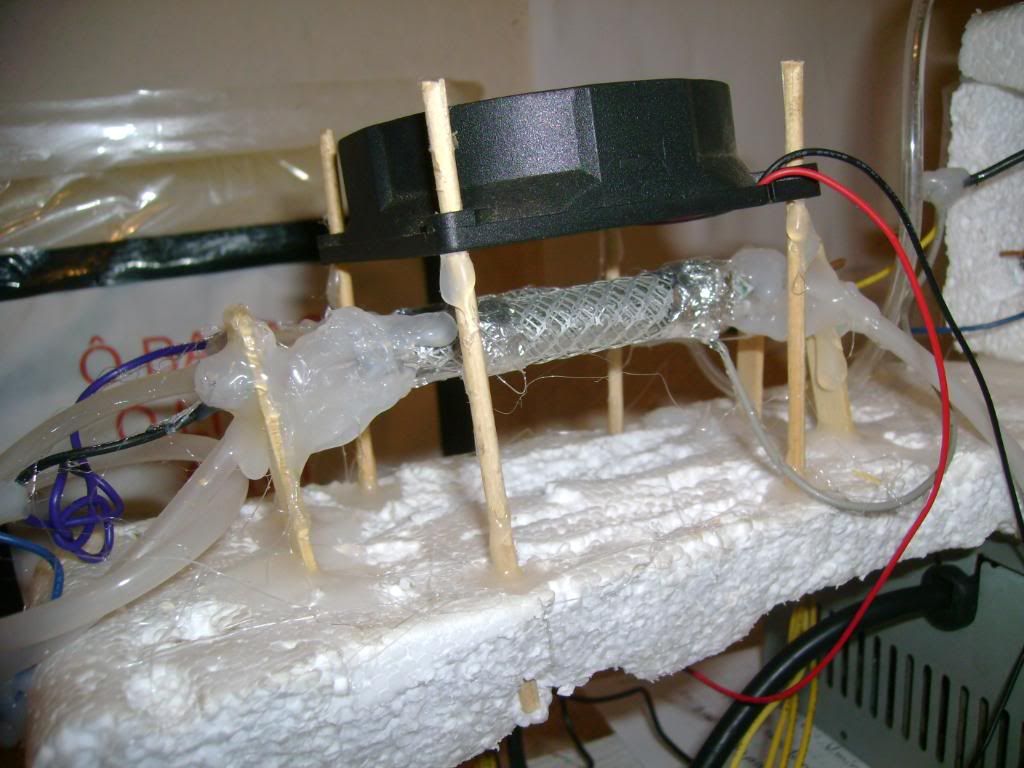





Video:
<object width="480" height="385"><param name="movie"
value="http://www.youtube.com/v/AicXCcNH_og?fs=1&hl=pt_BR"></param><param name="allowFullScreen"
value="true"></param><param name="allowscriptaccess" value="always"></param><embed
src="http://www.youtube.com/v/AicXCcNH_og?fs=1&hl=pt_BR" type="application/x-shockwave-flash" allowscriptaccess="always"
allowfullscreen="true" width="480" height="385"></embed></object>
The cooler was used to drop somewhat the temperature of the corona cell.. The higher the temperature, the lower the O3 yield..
For the same reason Ive used a lower wattage CFL circuit (9W, IIRC)..
The older flyback is better to use than the new (rectified), just by the fact that isnt rectified, since high frequency AC is desired...
"The secret of freedom lies in educating people, whereas the secret of tyranny is in keeping them ignorant."
|
|
|
The WiZard is In
International Hazard
    
Posts: 1617
Registered: 3-4-2010
Member Is Offline
Mood: No Mood
|
|
Ozone generators from Popular Science
http://tinyurl.com/279kqvk
http://tinyurl.com/2fmw67v
http://tinyurl.com/2vphf6j
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
chemical production of ozone
There are several methods to prepare oxone by chemical means. All routes seem to suffer from relatively poor yields.
From Metal Peroxides
28 grams of ozone per cubic metre of oxygen evolved,
by gently heating small quantities of powdered barium peroxide in eight times its volume of concentrated sulphuric
acid. Hydrogen peroxide is likewise formed in small quantities under these conditions.
i. (2)H2SO4 + (2)BaO2 --> (2)BaSO4 + (2)H2O + O2
ii. H2SO4 + BaO2 --> BaSO4 + H2O2
iii. (3)H2SO4 + (3)BaO2 --> (3)BaSO4 + 4H2O + O3
The same investigator showed that similar results wore
obtained with other peroxides, notably those of magnesium,
zinc, sodium, and potassium.
From Permanganate
Even better results can be obtained by the decomposition
and gentle dehydration of permanganic acid or potassium
dichromate,
Mn2O7 --> 2MnO2 + O3.
De la Coux ("L'Ozone," p67) states that oxalic acid can be likewise employed in the proportion of 10 gms. of permanganate to 15 gms. of oxalic acid,
and that 90 c.c. of oxygen containing 3 milligrams of ozone can be obtained from this mixture.
Satisfactory yields of ozone may also be obtained by cautious addition of barium peroxide to a solution of potassium permanganate in sulphuric acid,
of density 1.85
From Persulfate
By the thermal decomposition of the persulphates, small quantities of ozone can be obtained. 20 grams of dry freshly prepared ammonium persulfate are
mixed with 15 gms. of nitric acid in a small flask. The air is subsequently displaced by carbon dioxide, and the mixture cautiously raised to 65° to
70° C. The reaction, which is strongly exothermic, proceeds somewhat vigorously when once started, and the resulting oxygen, after removal of the
carbon dioxide, contains 3 to 5 per cent, of ozone and small quantities of nitrogen. Malaquin (" J. Pharm. Chem.," VII, 3, 329, 1911)
|
|
|
wpenrose
Harmless

Posts: 17
Registered: 17-6-2007
Member Is Offline
Mood: No Mood
|
|
Ozone Generator
Quote: Originally posted by hissingnoise  | | Ozone is an interesting oxidiser, but its synthesis is difficult and inefficient; pity it can't be formed by a contact process like the catalytic
oxidation of so2 on pt. gauze. |
The easiest way to make ozone is by either short wave UV acting on air, or a corona discharge through pure oxygen.
A water jacketed condenser can be stuffed with stainless steel wool, and the outer barrel of the condenser wrapped with stainless foil or copper. The
inside and outside are the two electrodes.
Pure oxygen can then be allowed to flow through the water jacket, which has the right tubulations for the job. The two electrodes are connected to a
10KV or 15KV neon sign transformer. (At this point, the process becomes very dangerous unless you have a suitably insulated closure for the whole
apparatus.)
This apparatus can generate ozone concentrations up to 30%, which are extremely dangerous on inhalation or eye exposure. I once got a blast of ozone
in the face that took three days to recover from. I was using a fume hood, too. But use a fume hood at all costs anyway.
If air is used instead of oxygen, nitrogen oxides are produced as well.
In a closed system, excess ozone can be absorbed by olive oil adsorbed on alumina or charcoal pellets, BUT the absorber will get very hot and can
catch fire if exposed too long.
Make no mistake, this is a dangerous system to work with, and deserves solid and thoughtful construction to avoid the hazards of
high-voltage electricity, ozone gas, and fire, not to mention the explosive products of many reaction products of ozone.
[Edited on 9-28-2011 by wpenrose]
|
|
|
AndersHoveland
Hazard to Other Members, due to repeated speculation and posting of untested highly dangerous procedures!
    
Posts: 1986
Registered: 2-3-2011
Member Is Offline
Mood: No Mood
|
|
Potassium persulfate is inexpensively available at pool stores. Why is this not an easy route to ozone? Chemical generation can be used to obtain
larger quantities of ozone than electric discharge can, an the reaction is much faster.
Ozone reacts with nitrogen dioxide to form dinitrogen pentoxide, a stronger nitrating agent than anhydrous nitric acid.
(2)NO2 + O3 --> N2O5 + O2
N2O5 is an explosive solid that slowly decomposes at room temperature. It reacts with water to form HNO3, and this method could potentially be used to
concentrate azeotropic nitric acid to anhydrous nitric acid- simply pass the inert gas containing ozone, and dry NO2, into the nitric acid to
concentrate it.
Ozone reacts with either chlorine or chlorine dioxide in water to form perchloric acid. If no water is present, red liquid Cl2O6 will form. It is a
sensitive explosive and highly corrosive, even attacking gold.
|
|
|
IrC
International Hazard
    
Posts: 2710
Registered: 7-3-2005
Location: Eureka
Member Is Offline
Mood: Discovering
|
|
Vulture I am clueless, what is xkJ ?
Mn2O7 + xkJ -> 2MnO2 + O3
"Science is the belief in the ignorance of the experts" Richard Feynman
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
| Quote: | | Why is this not an easy route to ozone? |
Because ozone is very thermally unstable and decomposes rapidly at above-ambient temperatures . . .
|
|
|
Lambda-Eyde
National Hazard
   
Posts: 857
Registered: 20-11-2008
Location: Norway
Member Is Offline
Mood: Cleaved
|
|
An arbitrary number of kilojoules.
This just in: 95,5 % of the world population lives outside the USA
You should really listen to ABBAPlease drop by our IRC channel: #sciencemadness @ irc.efnet.org
|
|
|
IrC
International Hazard
    
Posts: 2710
Registered: 7-3-2005
Location: Eureka
Member Is Offline
Mood: Discovering
|
|
Thank you. I hate it when I'm stupid, I was thinking Kilojoules but the X with no space before KJ had me thinking it may be some abbreviation for
something else.
"Science is the belief in the ignorance of the experts" Richard Feynman
|
|
|
| Pages:
1
2 |