chemchem
Harmless

Posts: 9
Registered: 19-3-2005
Location: land down under
Member Is Offline
Mood: happy jack
|
|
nickel chloride
hi ! if one wanted to synthesise nickel chloride ,what acids besides nitric will nickel metal disolve in, if any ? could one disolve it by an
electrochemical method with a dc power source in a common acid ?
possibly like how palladium can be disolved in hcl with dc currant? sounds to me as possible similar method ? thanks ////
|
|
|
Darkblade48
Hazard to Others
  
Posts: 411
Registered: 27-3-2005
Location: Canada
Member Is Offline
Mood: No Mood
|
|
Correct me if I'm wrong, I was always under the impression that nickel would readily displace the hydrogen in HCl to form NiCl.
|
|
|
BromicAcid
International Hazard
    
Posts: 3227
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
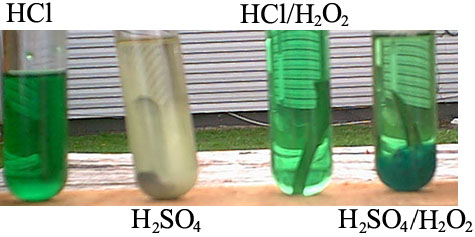
Nickel dissolves in HCl so slow you wouldn't even normally notice after an hour. Bubbling air though HCl will lead to faster oxidation. Adding
H2O2 to HCl will lead to faster oxidation. Performing electrolysis with nickel electrodes (the anode being submersed significantly further into the
solution then the cathode) in HCl will lead to rapid dissolution at about 12 V and 15A. Once you have as much nickel dissolved as you want (i.e. it
won't dissolve any more) it is difficult to evaporate a full solution of it because the nickel increases the boilng point significantly.
Attached is a picture from my book project for my attempts to dissolve nickel under several conditions, notice the attack in the H2SO4/H2O2 solution
[H2SO4 > 90%, H2O2 ~ 12%].
Edit: This was the result after 1 week at roughly 20 C, and if you're planning on working with palladium save yourself some pain and get some
nitric to dissolve it, you don't want to mess around with something that expensive, too much is lost and there is too great a possibility to mess
up.
[Edited on 4/13/2005 by BromicAcid]
|
|
|
chemchem
Harmless

Posts: 9
Registered: 19-3-2005
Location: land down under
Member Is Offline
Mood: happy jack
|
|
nickel chloride
thanks bromic.. mabee concerntrated hcl with a air bubbler and dask of hydrogen poroxide will disolve the metal not to slowly ?? then just boil off
hci water mix for your green nickel chloride crystals
|
|
|
BromicAcid
International Hazard
    
Posts: 3227
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Yeah, and while you're boiling down your HCl solution leave some solid nickel in there to dissolve some more while it's hot and active. A
little side note, anhydrous NiCl2 is yellow or maybe white (mine may not be dehydrated all the way...).
Related topics:
Nickel From Coins
http://67.15.145.24/~sciencem/talk/viewthread.php?tid=534
Nickel Sulfate
http://67.15.145.24/~sciencem/talk/viewthread.php?tid=2109
[Edited on 4/13/2005 by BromicAcid]
|
|
|
chemchem
Harmless

Posts: 9
Registered: 19-3-2005
Location: land down under
Member Is Offline
Mood: happy jack
|
|
thanks
thanks bromo muck appreciated info
|
|
|
chemchem
Harmless

Posts: 9
Registered: 19-3-2005
Location: land down under
Member Is Offline
Mood: happy jack
|
|
nickel anodes
i think the most fuss free method of obtaining nickel metal would be via the nickel anodes for welding .. shoul be able to pick them up from most
welding supplies shops ....
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
Alternatively, buy some Nickel oxide from a pottery supply and dissolve it in HCl.
Done it with HNO3, works very nicely.
PS: Chemchem, my spelling indicator is jagging wildely. Ever heard of CAPITALISATION?
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
chemchem
Harmless

Posts: 9
Registered: 19-3-2005
Location: land down under
Member Is Offline
Mood: happy jack
|
|
Nickel oxide from pottery shop ? Sounds good.. do you know of its use in potery ? Thanks for the reply .. Sorry about the punctuation on previous
posts >> Hope ive fixed that problem ! Thanks
|
|
|
chloric1
International Hazard
    
Posts: 1070
Registered: 8-10-2003
Location: GroupVII of the periodic table
Member Is Offline
Mood: Stoichiometrically Balanced
|
|
Colorant
Actually, chemchem, it is a colorant material. A raw material used to color pottery glazes. You will have to search online and it is not always easy
to find raw materials so you have to do some digging. Many pottery suppliers carry premixed glazes so they can make more money that way.
Fellow molecular manipulator
|
|
|
hodges
National Hazard
   
Posts: 525
Registered: 17-12-2003
Location: Midwest
Member Is Offline
|
|
One way to get nickel chloride in a reasonable amount of time with HCl is electrolysis. I once took an old Canadian coin that was mostly nickel and
made it the anode in HCl. HCl is a good conductor, so no problem getting current to flow even with a low voltage. I ended up with a dark green
solution. By keeping an excess of HCl very little nickel plates back out on the cathode.
Once completed you could neutralize the remaining HCl using NaOH and precipitate nickel hydroxide, which you could then use to make whatever salt you
want. Or you might be able to just evaporate the remaining HCl. .
|
|
|
aludel
Harmless

Posts: 6
Registered: 16-11-2004
Location: holland
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by BromicAcid
Related topics:
Nickel From Coins
http://67.15.145.24/~sciencem/talk/viewthread.php?tid=534
Nickel Sulfate
http://67.15.145.24/~sciencem/talk/viewthread.php?tid=2109
[Edited on 4/13/2005 by BromicAcid] |
Thanks for the info and pic!
Ah, in the good old days Dutch guilders and some other Dutch coins were made of nearly pure nickel! I used to dissolve them in HNO3 (taking care of
the fumes of course), precipitate the carbonate and dissolve that in H2SO4 to get nickel sulphate. Probably not pure enough for the ultra-black
stuff?? 
|
|
|
nitroglycol
Hazard to Self
 
Posts: 56
Registered: 28-10-2005
Location: close to the centre of North America
Member Is Offline
Mood: curious
|
|
Canadian quarters and dimes from before 2000 are essentially pure nickel (the new ones are nickel-plated steel, IIRC). I once attempted to dissolve
two quarters in 1 M HCl. After many months the solution had turned green but the coins were still there (damaged, but the images were still visible)
and the solution was still quite strongly acidic. I added more HCl (to bring it up to 2 M) but to no avail. Eventually I moved halfway across the
country and had to terminate the experiment. I'd like to try it with HNO3 but I think if I tried to buy
that stuff I'd have to answer a lot of questions about what I planned to blow up. Maybe I'll try Hodges' suggestion once I have my lab
set up.
|
|
|
Illegal Parkinson
Hazard to Self
 
Posts: 75
Registered: 2-10-2005
Member Is Offline
Mood: No Mood
|
|
I used not to have any interest in electrochemistry. However with chemical suppliers watching every item you buy im becoming increasing concious of
making chemicals that are for all intents and purposes "commercially available" if possible, and reserve the chem supplier for instances
where there is no way around it. There is a good section on electrochemistry in the book "Kings Chemistry" and Uncle Fester I believe was
also fond of it.
There has got to be a way of doing it. I seem to recall Rhodium had something on either his website or at the hive on this topic. Although the aim
there was to use palladium metal to make PdCl2, I think the same principles should apply. NiCl2 sounds like some tasty stuff 
|
|
|
chloric1
International Hazard
    
Posts: 1070
Registered: 8-10-2003
Location: GroupVII of the periodic table
Member Is Offline
Mood: Stoichiometrically Balanced
|
|
electrolysis
About 6 years ago I had a nickel electrode from one of the battery jar educational kits. It LOOKED like pure nickel but wasn't. I used it as an
anode in about 30% perchloric acid. As the solution got darker nad darker green, low and behold copper formed on the cathode!! I said "What the
hell?". Anyways, I just ran it until nickel began to plate and for all intents and purposes I purified my nickel 
[Edited on 10/31/2005 by chloric1]
Fellow molecular manipulator
|
|
|
Dr. Beaker
Hazard to Others
  
Posts: 132
Registered: 9-9-2005
Location: between the med red and dead
Member Is Offline
Mood: Shaken, not stirred
|
|
any other metal with higher reduction potential will be displaced by Ni.
example:
Cu+2 +Ni --> Ni+2 + Cu
the higher the differrence the better.
highschool chemistry...
|
|
|
nitroglycol
Hazard to Self
 
Posts: 56
Registered: 28-10-2005
Location: close to the centre of North America
Member Is Offline
Mood: curious
|
|
Yeah, but if you just drop a quarter in a copper sulphate solution, say, won't it accumulate a layer of Cu that will prevent further reaction?
|
|
|
Darkblade48
Hazard to Others
  
Posts: 411
Registered: 27-3-2005
Location: Canada
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by nitroglycol
Yeah, but if you just drop a quarter in a copper sulphate solution, say, won't it accumulate a layer of Cu that will prevent further reaction?
|
I've always noticd that when I drop a metal that will displace the copper from a copper sulfate solutio (i.e. magnesium, aluminum) that the
copper that comes out is not plated, but more of a spongy foam. This kinda just breaks away from the metal and copper continues being displaced by the
more reactive metal species.
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
BTW if you want a massive reducing agent, take an acidic CuCl2 solution and add aluminum. It'll get the solution boiling pretty quickly!
Cementation tends to leave a weak layer, but something so little above copper, like nickel, is going to take weeks to depost. Not to mention nickels
(the cheapest, most nickel-rich currency down here) are a solid solution of only 25% nickel, balance copper. As I posted months ago in this thread
(apparently), you're better off dissolving them with electricity, with copper plating on the cathode.
Tim
|
|
|
Darkblade48
Hazard to Others
  
Posts: 411
Registered: 27-3-2005
Location: Canada
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by 12AX7
BTW if you want a massive reducing agent, take an acidic CuCl2 solution and add aluminum. It'll get the solution boiling pretty quickly!
Tim |
Ironically, the reason why aluminum is so reactive in the presence of Cu+2 and chloride ions isn't too well understood
But it was fun nevertheless 
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
I've done that with acidic copper sulfate and zinc, it does get pretty hot and violent(nearly boils).
Also, nitroglycol, how could you forget Canadian nickels? pre 1982 canadian nickels are pure nickel. 1954 is nickel plated steel, and before that is
pure nickel again(till a certain year which I forget). If I could find my coin collecting book I could tell exactly the composition of all Canadain
coinage, I used to/still do do a bit of coin collecting.
|
|
|