PainKilla
Hazard to Others
  
Posts: 306
Registered: 29-4-2004
Member Is Offline
Mood: No Mood
|
|
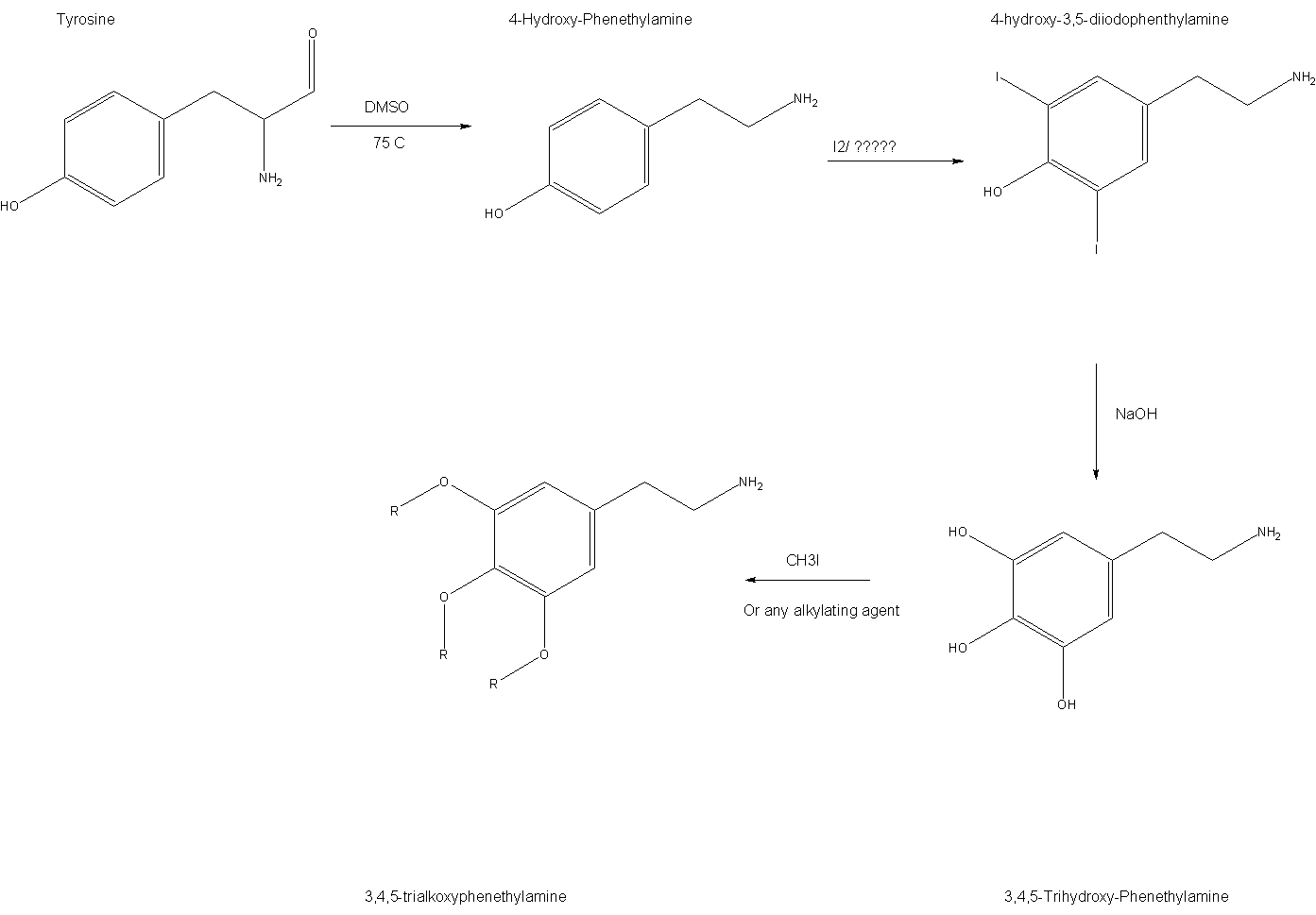
Theoretical Synthesis of 3,4,5-Alkoxy-PEA
Hehe, 3,4,5-Alkoxy-phenethylamines are very interesting and illusive compounds. My friend and I have recently been discussing a route to such
compounds and anologues. The idea was to start with tyrosine, a cheap and very easy to get vitamin supplement. The rest is pretty much OTC, yet
involved some interesting chemistry and techiniques, so I quite enjoy trying to see if it can be done.
Being that am still a noob at organic chemistry, mainly because my teachers are idiots (and that I dont officialy start learning it for another 3
years.)
My proposed method for this would be to start with tyrosine, or 4-Hydroxy-phenethylbenzoic acid amine? I have not too much idea on organic
nomenclature of the more complex aromatics. Anyway, this is decarboxalated via a few methods, though the most promising and cheap seems to DMSO, which
is suited perfectly for this task. The product yield, being tyramine (4-hydroxyphenethylamine), is probably nearly 100%. This is then, halogenated
with Br or I, (for me I because it is easier.) This forms 4-hydroxy-3,5-diiodophenethylamine, most likely with a few percent of the mono and tri/tetra
iodo deratives. However I am told that the latter is unlikely, and with long enough reaction the mono will be be made into the di. This is treated
with NaOH forming 3,4,5-trihydroxyphenethylamine. From here one can ethylate, methylate, regardless and be left with thier desired product. I plan to
methylate, using CH3I which seems the safest and easiest to attain.
Here is the synopsis: Tyrosine--DMSO/50C->Tyramine ---Halogenation-->4-hydroxy-3,5-dihalophenethylamine ---NaOH-->
3,4,5-Trihydroxyphenethylamine --alkylate--->3,4,5-triAlkoxyphenethylamine
However, I do have a few questions, and they might be noob I dont know  . .
1. Why does the OH cause the Iodines to go onto the para/ortho positions?
2. What are suitable iodination agents, from what I have found, I2/nitric.... I2/H2O2 (only heard never seen used "officialy"..... I2/Lewis
Acid (can it be any lewis acid, and also a mechanism would be most appreicated)?
3. Do any special solvents need be used, such as DMF for methylation.... or, can everything be done straight up, without "special" solvents?
4. Could someone please explain how I would setup a vacuum for getting rid of the DMSO? (Ill be using an aspirator)
Thank for your help it is much appreciated... I will report yields and all that once I do this. I will be doing it over a ling time as a side project
so, it may take a while. I will be decarboxalating the tyrosine in a week or two however. If I can find an easy way to iodinate, then that and the
NaOH will be done within a month. CH3I will have to wait until I assemble my distilaltion set.
[Edited on 5-5-2005 by PainKilla]
|
|
|
PainKilla
Hazard to Others
  
Posts: 306
Registered: 29-4-2004
Member Is Offline
Mood: No Mood
|
|
Here is the picture, its attached.
|
|
|
sparkgap
International Hazard
    
Posts: 1234
Registered: 16-1-2005
Location: not where you think
Member Is Offline
Mood: chaotropic
|
|
Ah, going for mescaline analogues, PainKilla? 
Anyway, to answer your questions:
1. The -OH group tends to make the ortho- and para- positions more attractive to electrophiles due to increased electron density.
Hence, you get 4-hydroxy-3,5-diiodophenethylamine after iodination.
2. The Sandmeyer reaction is the textbook way of introducing -I into aromatic rings. I don't remember any other routes, but maybe the former
"bees"  in this forum may remember something. But why not bromine?
Handling? Ferric bromide is cheap. in this forum may remember something. But why not bromine?
Handling? Ferric bromide is cheap.
3. What solvents do you have there? IMHO, any decent nonpolar solvent that won't be alkylated by MeI should do.
4. I'll be referring you to the vacuum distillation experts in this forum... 
Hope this helps.
sparky (^_^)
P.S. I believe there was a thread in Organic Chemistry concerned with amino acid decarboxylations. You might want to search for that.
"What's UTFSE? I keep hearing about it, but I can't be arsed to search for the answer..."
|
|
|
PainKilla
Hazard to Others
  
Posts: 306
Registered: 29-4-2004
Member Is Offline
Mood: No Mood
|
|
Well, I personally am going for the methoxy analogue  . Thanks for your reply. . Thanks for your reply.
1. Ok sick, thanks.
2. I thought the sanmeyer reaction is the diazotization or w/e, substitution reaction.... but I have no NH2 besides on the PEA... and I dont want to
change it  . I dont really want to use Br because its more work for me to get,
whereas I can already get Iodine cheaply, and already need it for CH3I and I might as well just order more. I was thinking a nice direct I2/H2O2...if
this works. . I dont really want to use Br because its more work for me to get,
whereas I can already get Iodine cheaply, and already need it for CH3I and I might as well just order more. I was thinking a nice direct I2/H2O2...if
this works.
3. ATM, I have nothing  . I am hoping to get some CCl4 though via chlorination of
chloroform. This should be nicely yielding and well worth it. I hate how everyone over exagerates its toxicity though. . I am hoping to get some CCl4 though via chlorination of
chloroform. This should be nicely yielding and well worth it. I hate how everyone over exagerates its toxicity though.
4. OK  . .
Much thanks.. I cant wait for my tyrosine to come in. Time for school  . .
|
|
|
Sergei_Eisenstein
Hazard to Others
  
Posts: 290
Registered: 13-12-2004
Location: Waziristan
Member Is Offline
Mood: training
|
|
Amongst several things that won't work as you lined out, there is the alkoxylation of 3,4,5-trihydroxyphenethylamine with iodomethane, as it will
not only alkylate the phenolic OH, but also the amine (can form quaternary salts).
|
|
|
kyanite
Hazard to Self
 
Posts: 86
Registered: 30-6-2004
Location: is not important!
Member Is Offline
Mood: lactamic
|
|
I don't know if this matters for mescaline, etc, but heads up- the amino acids are of the levro isomer, and so your product will be levro too.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Kyanite, mescaline has no chiral centers and hence no enantiomers. Neither does tyramine, so the chirality of the amino acid, tyrosine in this case,
does not matter at all.
Actually, not only several things, but none of the proposed reactions would work. Except maybe for the tyrosine decarboxylation, which is however a
very difficult one to perform (nearly impossible for someone who does not have enough laboratory experience).
Besides, mescaline is illegal to make and poses in most countries. Escaline and many other homologues, considerably more potent than mescaline, are
not.
|
|
|
a123x
Hazard to Self
 
Posts: 87
Registered: 12-1-2003
Member Is Offline
Mood: No Mood
|
|
So far I've been having a fairly good time of decarboxylating the tyrosine by refluxing it in a high BP solvent with some ketone catalyst. It
certainly seems to be releasing CO2 although the reaction does seem a bit slow.
Why do you think that all of these reactions won't work. I understand the possible problems with alkylating the amine which is why I intend to
react the iodo(or maybe bromo) compound with an alkoxide to directly get the desired alkoxy group. This still will require alkylation of the hydroxide
with an alkyl halide but it will require less alkyl halide and so I hope that this will help prevent the amine from being too affected. Other than
that I don't see what else won't work. I imagine that I could easily be missing something.
|
|
|
Kinetic
Harmless

Posts: 45
Registered: 31-12-2004
Member Is Offline
Mood: fine
|
|
Why? Because without references all this is nothing but idle speculation. Sergei_Eisenstein and Nicodem are right, and both are knowledgeable enough
for you to take their word on the subject.
a123x, have you actually obtained (and characterised) any product from your atttempted decarboxylation? Do you have any references for the
nucleophilic substitution of methoxide on a similar nucleus to 3,5-dihalotyrosine?
Both of you should search for related references for each step of any proposal you make. This way you are more likely to get an involved reply from
someone who knows what they're talking about, and you will also be able to answer many of your own questions as you go along.
I used to make posts like this at the Hive years ago and never understood why nobody took any notice. Now I do. People are not interested in somebody
putting together a proposal which they hope might work but actually have no idea if it will.
You will learn so much more if you take the time to seriously research and reference every step. If you cannot find any related references, there is
often a good reason for the fact. | Quote: | | This still will require alkylation of the hydroxide with an alkyl halide but it will require less alkyl halide and so I hope that this will help
prevent the amine from being too affected |
It doesn't work like that. You will be unable to selectively
alkylate a phenol in the presence of a free amine. I could find no reports of the O-alkylation of tyrosine or, for example, 4-hydroxybenyzylamine or
4-aminophenol; the same will apply to your substrate.
Protection would be a must if you used this method, if you were to ever get this far.
Tyrosine has been 3,5-dihalogenated according to the literature. For diiodination, see Patent DE259193
I don't think anyone should make mescaline without a license. Not because I believe it is wrong, but because of the consequences for your life if
you get caught. Analogues such as escaline are in most cases just as easy to make, and will cause you considerably less trouble should you get caught
with them. There are exceptions of course: in certain countries most analogues, escaline included, are as strictly controlled as mescaline itself.
|
|
|
PainKilla
Hazard to Others
  
Posts: 306
Registered: 29-4-2004
Member Is Offline
Mood: No Mood
|
|
Well then, thank you everyone for your replies. I don't think stuff should not be made because its illegal. It's not I planned to sell it or
anything like that. I am doing more for the chemistry than the product (which I can get easier than getting any of the solvents or pretty much
anything for this anyway.)
I have no idea where to start, i mean reference-wise, searching patents.... I still dont know where to start. On top of that, I cant get any formal
organic chemistry knowledge until at least another year or two. Reading books doesnt quite cut when going over diastereomers and entiomers etc... And
if i skip that I miss out on a large amount...etc and this is with a few topics. I can't ask any of the teachers in my school because they dont
know what cis/trans is so.... well you get my point.
I looked into the thing with the amine and do indeed see that it would be hard to methylate, and maybe iodinate. It can still be tried though. If you
dont mind me asking, do you know what possible products could form? Im sure I can make something interesting  . .
Also, is there any good place to learn organic chemistry? I dont even feel very comfortable discussing with my teacher (who is an idiot anyway)
because of the "meth lab" thing.
Thanks for your replies then  . .
|
|
|
Sandmeyer
National Hazard
   
Posts: 784
Registered: 9-1-2005
Location: Internet
Member Is Offline
Mood: abbastanza bene
|
|
| Quote: | Originally posted by PainKilla
Also, is there any good place to learn organic chemistry? |
Dusty basements, libraries, the dustier the better...
Vitus even keeps his women there:


[Edited on 7-5-2005 by Sandmeyer]
|
|
|
sparkgap
International Hazard
    
Posts: 1234
Registered: 16-1-2005
Location: not where you think
Member Is Offline
Mood: chaotropic
|
|
That was very funny, Sandmeyer. 
PainKilla:
"...if you don't mind me asking, do you know what possible products could form? I'm sure I can make something interesting..."
How about trying to make an OTC phase-transfer catalyst? 
Tsk, I just knew someone would mention mescaline's unlawful status.
And BTW, patent searches (at least in the U.S.) can be done via www.uspto.gov
sparky (~_~)
"What's UTFSE? I keep hearing about it, but I can't be arsed to search for the answer..."
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
PainKilla, it's true, there is nothing better than libraries to search for journal papers. The difficult part is to find relevant references.
Unfortunately most libraries only give you access to the "not-electronic" versions of Beilstein and Chemical abstracts (at least where I
live). But searching trough those can make you hate them very soon.
Recently there is also a trial version of a wannabe pseudo-Beilstein clone where you can register on trial basis for a week:
http://www.spresi.com/
(it does not give you abstracts though, and it is also full of errors, but at least you can search by structure and reactions)
There is also a search engine for the papers accessible online. Needless to say that most of this papers require login, but at least you get the
reference and abstract:
http://www.scirus.com/
Also, most libraries that have at least one journal subscribtion from Elsevier publisher will have free online access by (IP recognition) to all their
journals (which are many). Download them from such a library or university. There are also other publishers who offer this kind of access. Search the
internet for them. There are some large compiled lists available for such sources.
http://www.sciencedirect.com/
There is also the famous PubMed database:
http://www.ncbi.nlm.nih.gov/entrez/query.fcgi
there are other sources as well, but search for them yourself as well.
|
|
|