| Pages:
1
..
23
24
25
26
27
..
31 |
caterpillar
Hazard to Others
  
Posts: 472
Registered: 8-1-2012
Member Is Offline
Mood: No Mood
|
|
What Google exists for? Try "Jared Ledgard preparatory manual of explosives" and you'll get dozens of links. I do not remember, where I downloaded it
from (but for free!). But be careful: this famous book contents really much BS. Read at this very forum "the worst book, ever written". For example,
this wise guy wrote, that ammonium picrate is very sensitive and used as a primer.
Women are more perilous sometimes, than any hi explosive.
|
|
|
Bot0nist
International Hazard
    
Posts: 1559
Registered: 15-2-2011
Location: Right behind you.
Member Is Offline
Mood: Streching my cotyledons.
|
|
papaya.
Seriously, just follow roscos workup with ASA. The extra amount of. sulfuric (93%) aids in sulfanation of ASA, keeps reaction liquid, and the extra
bit of water is benifitial to the sulfanation and nitration. Follow the temps and additions to the tee.
,
No more spoonfeeding. Go run the reaction, follow the writeup, and mind the NO<sub>x</sub>.
[Edited on 2-12-2013 by Bot0nist]
U.T.F.S.E. and learn the joys of autodidacticism!
Don't judge each day only by the harvest you reap, but also by the seeds you sow.
|
|
|
papaya
National Hazard
   
Posts: 615
Registered: 4-4-2013
Member Is Offline
Mood: reactive
|
|
Thanks guys, found Ledgard - should I trust someone who presents oxidation state and vallence as they were the same thing? I trust more "official"
literature like Urbanski, etc (also some russian authors). Anyway one more question - Rosco describes synthesis that took him 5 hours, is this really
the case for small batches (like 5g) or after one gradually adds nitrate and foaming is over + some 30 minutes at 110 °C is enough ? And pls, don't
call this "spoonfeeding" I really checked literature before asking, here in forum one expects to hear real experience, a "recipe" is easy to find. I
will see when (if) I try this.
|
|
|
Bot0nist
International Hazard
    
Posts: 1559
Registered: 15-2-2011
Location: Right behind you.
Member Is Offline
Mood: Streching my cotyledons.
|
|
Addition times should be adjusted in order to keep the temps as he recorded them in his larger scale writeup. Temps are vital to this nitration, in my
experiance.
[Edited on 3-12-2013 by Bot0nist]
U.T.F.S.E. and learn the joys of autodidacticism!
Don't judge each day only by the harvest you reap, but also by the seeds you sow.
|
|
|
caterpillar
Hazard to Others
  
Posts: 472
Registered: 8-1-2012
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by papaya  | | Thanks guys, found Ledgard - should I trust someone who presents oxidation state and vallence as they were the same thing? I trust more "official"
literature like Urbanski, etc (also some russian authors). Anyway one more question - Rosco describes synthesis that took him 5 hours, is this really
the case for small batches (like 5g) or after one gradually adds nitrate and foaming is over + some 30 minutes at 110 °C is enough ? And pls, don't
call this "spoonfeeding" I really checked literature before asking, here in forum one expects to hear real experience, a "recipe" is easy to find. I
will see when (if) I try this. |
I described here my own experience. I'd made some mistakes before I found right "recipe", but I think this sort of experience has no interest. Yeah,
temperature regime is just that thing which you have to keep under control, otherwise runaway is possible.
Women are more perilous sometimes, than any hi explosive.
|
|
|
Bot0nist
International Hazard
    
Posts: 1559
Registered: 15-2-2011
Location: Right behind you.
Member Is Offline
Mood: Streching my cotyledons.
|
|
Not just cascading runaway. but also undesirable oxidation products, resinous gunk, and low yields if the temperature is controled. poorly throughout
the reaction.
U.T.F.S.E. and learn the joys of autodidacticism!
Don't judge each day only by the harvest you reap, but also by the seeds you sow.
|
|
|
papaya
National Hazard
   
Posts: 615
Registered: 4-4-2013
Member Is Offline
Mood: reactive
|
|
Thanks a lot, temperature is important, though what if one conducts synthesis on the boiling water bath (a little lower temperature than needed), and
only heat higher at the very end? Also, any notes on salicylic acid usage instead of ASA, how to adjust quantities then, just on 1 to 1 molar basis ?
Hope not bored everybody yet.
|
|
|
Bot0nist
International Hazard
    
Posts: 1559
Registered: 15-2-2011
Location: Right behind you.
Member Is Offline
Mood: Streching my cotyledons.
|
|
The ASA sulfanation will break the ester anyway. I used a simmering brine bath to heat the sulfuric acid during ASA additions, and again to keep
the.nitration heat up durring the first small additions. Then it may have to be removed from the bath as the exotherm from nitrate salt.additions
picks up. Good mag stirring is vital. Think vortex...
U.T.F.S.E. and learn the joys of autodidacticism!
Don't judge each day only by the harvest you reap, but also by the seeds you sow.
|
|
|
papaya
National Hazard
   
Posts: 615
Registered: 4-4-2013
Member Is Offline
Mood: reactive
|
|
Thing is I found rather pure salicylic acid (reagent), so don't want to bother with tablets, etc...
|
|
|
Bot0nist
International Hazard
    
Posts: 1559
Registered: 15-2-2011
Location: Right behind you.
Member Is Offline
Mood: Streching my cotyledons.
|
|
Just run it as if it were ASA. Sulfanate and nitrate, and record your results for possible improvments.
U.T.F.S.E. and learn the joys of autodidacticism!
Don't judge each day only by the harvest you reap, but also by the seeds you sow.
|
|
|
caterpillar
Hazard to Others
  
Posts: 472
Registered: 8-1-2012
Member Is Offline
Mood: No Mood
|
|
Go ahead. Your own experience is the most valuable. And learn chemistry- pure theory is useful thing too.
Women are more perilous sometimes, than any hi explosive.
|
|
|
roXefeller
Hazard to Others
  
Posts: 463
Registered: 9-9-2013
Location: 13 Colonies
Member Is Offline
Mood: 220 221 whatever it takes
|
|
I found to avoid a great deal of the NOx emissions was to split the sulfuric acid in two, the first part reacts with the ASA, while the second part,
roughly in excess of stoichiometric amounts, was mixed with the nitrate salt. Then this is added the same way as the nitrate salt originally. There
was a sweet spot for the addition rate that could be dialed in with the sep funnel. Beyond this spot would bring about the fumes again, but still not
as bad as before.
|
|
|
barley81
Hazard to Others
  
Posts: 481
Registered: 9-5-2011
Member Is Offline
Mood: No Mood
|
|
If it helps, I typeset/edited Rosco's original procedure (Dec 9, 2003, E&W forum). Note that this is <b>not the same</b> as the
original. Any corrections or improvements would be appreciated. LaTeX and PDF files are both attached.
Has this been done already? If so, then *oops.*
Attachment: roscopicric.pdf (89kB)
This file has been downloaded 5415 times
Attachment: roscopicric.tex (7kB)
This file has been downloaded 1314 times
[Edited on 14-12-2013 by barley81]
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
For a long time I was off and on questioning the purity of the picric acid I was producing from ASA. Here is a couple of articles I found a while back
which helped convince me that picric acid is the usual and only product from the direct sulfonation and nitration of ASA or salicylic acid.
Attachment: 4-Sulfo Salicylic Acid Nitration.pdf (1.1MB)
This file has been downloaded 1103 times
Attachment: 4-Sulfo Salicylic Acid Synthesis.pdf (424kB)
This file has been downloaded 683 times
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
For those who may be looking for the other thread referenced for this synthesis
picric acid from ASA , NaNO3 and 92% H2SO4
http://www.sciencemadness.org/talk/viewthread.php?tid=4457&a...
Also attached are the ordenblitz images which have dead links
Attachment: ordenblitz pictures picric acid synthesis.pdf (663kB)
This file has been downloaded 663 times
|
|
|
Hawkguy
Hazard to Others
  
Posts: 326
Registered: 10-10-2014
Location: British Columbia (Canada eh!)
Member Is Offline
Mood: Body is Ready
|
|
2, 4, 6 – Trinitrophenol [Picric Acid]
Picric Acid is a light yellow solid of bitter taste, hence its name. It was discovered in 1742 (earliest documentation), although it could have
potentially been synthesised earlier by the nitration of organic materials. Picric Acid’s applications remained solely to use as a chemistry reagent
and as a dye until 1830, when its properties as a secondary explosive were realized. The First World War saw extensive use of Trinitrophenol.
Explosive action ceased however, because of its reactivity towards metals, forming unstable or hygroscopic salts. By the time World War Two came
around, Picric Acid was only used as a Picratol precursor. Today the explosive is obsolete as to military application, but is sometimes used in
fireworks for whistle mixes.
Preparation
Although 2, 4, 6 – Trinitrophenol was most often synthesized from Phenol, it is much easier to do so from Acetylsalicylic Acid. This is also a lot
safer, as free Phenol, a potent carcinogen, is not handled directly through the ASA route.
- 6 grams of pure Acetylsalicylic Acid are dissolved in 60ml of 18M Sulphuric Acid at 20°C. The solution is heated over the course of 3 minutes to
110°C, and the Acetylsalicylic Acid will hydrolyse to Acetic and Salicylic Acid. The colour of the solution will change from clear, to yellow, to
black. The Salicylic Acid will decarboxylate to Phenol and Carbon Dioxide, and Phenylsulphonic Acid will be formed.
- Potassium Nitrate is added in small portions over the course of the next 10 minutes. With every addition, the temperature will rise to 120°C. It is
important to keep the temperature below 130°C, so a thermometer must be carefully monitored at all times. At this point the Phenylsulphonic Acid is
undergoing a triple nitration to produce water, Sulphuric Acid, and 2, 4, 6 – Trinitrophenol. The temperature is dropped, and the colour will become
an orange – red.
- After 5 minutes since the solution was removed from heating, it can be added to 300ml crushed ice, and a yellow precipitate will immediately form.
This is the product, and it can be collected.
- [Purification] The product must undergo two water washes, rinses with water to remove acid and other residual contaminants. As well, a
recrystallization from Ethyl Alcohol helps remove insoluble contaminants.
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
| Quote: |
By the time World War Two came around, Picric Acid was only used as a Picratol precursor. Today the explosive is obsolete as to military application,
|
I beg to differ.
During WWII, the Japanese used picric acid in shell fillers extensively, and the Germans used picric acid as a booster charge for some rather large
TNT charged ordnance (500kg TNT parachute deployed naval mines with 1kg pelletized picric acid as a booster?!).
So... What do you have to contribute from personal experience.
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
caterpillar
Hazard to Others
  
Posts: 472
Registered: 8-1-2012
Member Is Offline
Mood: No Mood
|
|
Shellite, what was used during WWII in large caliber shells by Britons, was as a matter of fact picric acid with few percents of something like
nitrophenole. Ammonium picrate ("dunnite" or compound "D") was used in anti-tank shells due to its low sensibility (lower than of the TNT).
Women are more perilous sometimes, than any hi explosive.
|
|
|
Hawkguy
Hazard to Others
  
Posts: 326
Registered: 10-10-2014
Location: British Columbia (Canada eh!)
Member Is Offline
Mood: Body is Ready
|
|
Sorry about that... Yeah you're right then.. I totally though that Picric was starting to be replaced by more modern nitrated explosives by then...
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Quote: Originally posted by Hennig Brand  | For a long time I was off and on questioning the purity of the picric acid I was producing from ASA. Here is a couple of articles I found a while back
which helped convince me that picric acid is the usual and only product from the direct sulfonation and nitration of ASA or salicylic acid.
|
This is the article I think will be most helpful. See first sentence at top of second page of article journal page 2040. This applies to the case
involving 5-sulfosalicylic acid
Datta and Varma, J. Amer. Chem. Soc., 1919, 41, 2039
(article attached)
Attachment: JACS Vol 41 pg2039.pdf (414kB)
This file has been downloaded 638 times
[Edited on 30-3-2015 by Rosco Bodine]
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Thanks for locating that. We have a nice collection of old articles now (pieces of the puzzle).
It is the first paragraph from the first article of the two that I posted above, where your article is referenced, which immediately got me very
interested at the time.
"It has been found by the previous workers that the sulphonic acid group
in either 3-sulpho- or 5-sulpho-salicylic acid can easily be substituted by
the nitro group, and if the reaction is carried further, the -COOH group is
removed and picric acid is the usual and the only product. Thus Datta
and Varma obtained picric acid by the action of nitrous gases on 5-sulphosalicylic
acid. Meldrum and Hirwe obtained 3 : 5-dinitrosalicylic acid by
controlled nitration of 5-sulphosalicylic acid."
3 : 5-dinitrosalicylic acid is the intermediate just before decarboxylation and addition of the last nitro group forming picric acid.
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
The sulfonate group is more stable than the carboxyl so the "steering effect" on substitution by the first entering nitro will I believe cause the
decarboxylation and substitution of the nitro to occur for the first entering nitro group.
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
I am just an amateur experimenter, not a professional chemist, but I did asked Philou to explain this to me well over a year ago now and this is what
I was told:
"Salicylic acid is very closely related to phenol and the hydroxy group is a very strong activator in ortho and para while the carboxy is an activator
in meta... both effects are thus additive and favoring ortho and para position to the OH.
Once those two places are nitrated, the nitro groups are dropping the CO2 away from the aromatic ring and the rest of the reaction follows the course
of normal nitration of 2,4-dinitrophenol"
5-Sulphosalicylic acid structure taken from Wiki.
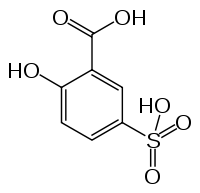
I think you must be forgetting, or just recently saw something that made you re-question some of this. The following was taken from "Rosco's Good Old
Country Recipe for TNP":
"When about 2/3 of the sodium nitrate has been added, the reaction product is largely dinitrosalicylic acid, which will acquire a third nitro group
while evolving carbon dioxide and thereby be converted to picric acid as the reaction proceeds."
[Edited on 30-3-2015 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
It isn't really a substantive issue what is the mechanism, or if different paths are occurring simultaneously when all reactions in sum lead to the
same 2,4,6-trinitrophenol as the end result in the same reaction mixture. All roads lead to Rome there.
For the case of phenol the hydroxyl on the ring strongly promotes ring substitution, much more than a ring hydrogen on ordinary benzene, but an added
sulfonate group hinders further substitution relative to a ring hydrogen. For salicylic acid the carboxyl further hinders substitution even more than
a sulfonate. In the case of 5-sulfosalicylic acid there is the promoting hydroxyl of phenol and BOTH the inhibiting sulfonate and further there is
the even more inhibiting carboxyl.
My interpretation is the inhibiting groups would themselves be subject to substitution preferentially to the unsubstituted #6 position ring hydrogen
which would be nitro substituted dead last if the nitration was steered according to my guess what happens. My understanding may be wrong but I would
have supposed the carboxyl would be first to nitrate, the sulfonate would be second, agreeing with the intensity which they hinder substitution
relative to a ordinary ring hydrogen, making the plain ring hydrogen at 6 the last to nitrate.
That is my guess how the reaction may proceed. Whether the guess is correct or not I don't know. I would trust what Nicodem may say about this more
than Philou 
BTW I do not even like the naming convention for 5-sulfosalicylic acid
When I look at the molecule I think of this name:
2-carboxyl-para phenolsulfonic acid
[Edited on 30-3-2015 by Rosco Bodine]
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
As long as all roads do in fact lead to Rome I am satisfied, which was my main interest before when I decided to do a little investigating. I found
the naming of 5-sulfosalicylic acid a bit confusing myself.
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
| Pages:
1
..
23
24
25
26
27
..
31 |