| Pages:
1
..
24
25
26
27
28
..
31 |
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Quote: Originally posted by Hennig Brand  |
I think you must be forgetting, or just recently saw something that made you re-question some of this. The following was taken from "Rosco's Good Old
Country Recipe for TNP":
"When about 2/3 of the sodium nitrate has been added, the reaction product is largely dinitrosalicylic acid, which will acquire a third nitro group
while evolving carbon dioxide and thereby be converted to picric acid as the reaction proceeds."
|
Yes I am questioning what I said earlier after thinking about how freely the decarboxylation foaming was occurring during the first third of the
nitrate additions. I think I also made that observation. Reflecting on what I had observed has caused me to reevaluate that conclusion about what I
observed, since most of the carboxyl group would have been decomposed early in the reaction.
Subsequent experiments will be done at a slightly different reaction condition and a thinner mixture or different temperature ramping. I think the
conditions can also change what reaction course is the one which predominates during different times of the course of the reaction, and I think in
different proportions all three possible nitro substitution reactions are occurring simultaneously. It could result in an improved synthesis to test
variations and compare results.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
additonal references
Attached are some additional references related to sulfonation and nitration of salicylic acid. I think the only relevant mention about the
sulfonation is on the first page of the article. Also attached is some general information about salicylic acid from Von Richter which pointed to
those articles, which are in German.
Off topic but worth mentioning ....
I found it interesting also that methyl salicylate reacts readily with aqueous hydrazine to form a hydrazide of salicylic acid identified by Von
Richter which may further be reacted with nitrous acid to form an azide. This could have interest as an obscure possibly useful route to azide.
Attachment: Nitro-sulfo-salicylic Acid.pdf (438kB)
This file has been downloaded 617 times
Attachment: Sulfonation of Salicylic Acid.pdf (606kB)
This file has been downloaded 612 times
Attachment: Salicylic Acid Pages from Von Richter.pdf (212kB)
This file has been downloaded 735 times
Attachment: Salicylic Acid Hydrazide.pdf (239kB)
This file has been downloaded 725 times
|
|
|
papaya
National Hazard
   
Posts: 615
Registered: 4-4-2013
Member Is Offline
Mood: reactive
|
|
Quote: Originally posted by Rosco Bodine  |
I found it interesting also that methyl salicylate reacts readily with aqueous hydrazine to form a hydrazide of salicylic acid identified by Von
Richter which may further be reacted with nitrous acid to form an azide. This could have interest as an obscure possibly useful route to azide.
|
Very interesting, could this be done also with benzoate instead of salicylate ? Also, is it at all necessary to have methyl ester or plain acid can
substitute it?
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by papaya  | Quote: Originally posted by Rosco Bodine  |
I found it interesting also that methyl salicylate reacts readily with aqueous hydrazine to form a hydrazide of salicylic acid identified by Von
Richter which may further be reacted with nitrous acid to form an azide. This could have interest as an obscure possibly useful route to azide.
|
Very interesting, could this be done also with benzoate instead of salicylate ? Also, is it at all necessary to have methyl ester or plain acid can
substitute it? |
This is an old known reaction initially found with hippuric acid hydrazide...an old way to make HN3
Ar-CO-OAlk + H2N-NH2 --> Ar-CO-NH-NH2 + Alk-OH
Ar-CO-NH-NH2 + HONO --> Ar-CO-NH-NH-N=O + H2O
Ar-CO-NH-NH-N=O <--> Ar-CO-NH-N=N-OH --> Ar-CO-N3 + H2O
Upon basic hydrolysis the aryl azide will generate an arylate and azide salt.
Yes it will work with benzoic acid. The ester is a convenient way to get the hydrazide easily (just like amide from NH3 and esters); this will not
work directly from benzoic acid.
Methyl or ethyl esters are easier to get and the resulting methanol/ethanol easy to evaporate/separate.
The hydrazide is crystalline compound while the ester is usually liquid, just like hydrazine...so synthesis is pretty straightforward...
The aryl azide will degrade upon thermolysis via Curtius' degradation into isocyanate and after hydrolysis a convenient way to amines, it works also
with the alkylic familly!
[Edited on 31-3-2015 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
This scheme is something that may fit my idea about synthesis of azide in decent yield being possible using aqueous solutions. Digesting the aryl
azide with lime water should precipitate a low solubility calcium salt of the carboxylic acid and leave highly soluble calcium azide in the filtered
solution. Reaction with sodium carbonate would precipitate calcium carbonate and leave highly soluble sodium azide in solution, evaporated to obtain
crystallized sodium azide.
Could simply reacting the hydrazine product with MEK, or acetone azine, mixed with methyl salicylate, then produce the same salicylic acid hydrazide
as the product?
[Edited on 31-3-2015 by Rosco Bodine]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Quote: Originally posted by papaya  | Quote: Originally posted by Rosco Bodine  |
I found it interesting also that methyl salicylate reacts readily with aqueous hydrazine to form a hydrazide of salicylic acid identified by Von
Richter which may further be reacted with nitrous acid to form an azide. This could have interest as an obscure possibly useful route to azide.
|
Very interesting, could this be done also with benzoate instead of salicylate ? Also, is it at all necessary to have methyl ester or plain acid can
substitute it? |
Yes it is necessary to have the ethyl or methyl ester of the carboxylic acid to form the hydrazide which is different from the simple acid salt of
hydrazine. Hydrazine Salicylate mp 106C
is a different compound from Salicylic Acid Hydrazide mp 147C (corr. 150-151C) The salicylate of hydrazine is simply hydrazine coupled with salicylic
acid. This salt has 2 more hydrogen atoms than the different molecule which is the hydrazide of salicylic acid.
https://books.google.com/books?id=JGJFAQAAIAAJ&pg=PA831&...
It is interesting that a mixture of methyl salicylate and hydrazine hydrate microwaved gently 450W for two minutes gives a 90% yield of the hydrazide
compared with a 65% yield gotten by refluxing the reaction mixture for 1 hour.
See attached article
Attachment: Rapid Solvent-free Synthesis of Aromatic Hydrazides under Microwave Radiation.pdf (247kB)
This file has been downloaded 688 times
[Edited on 1-4-2015 by Rosco Bodine]
|
|
|
papaya
National Hazard
   
Posts: 615
Registered: 4-4-2013
Member Is Offline
Mood: reactive
|
|
I was thinking of synthesizing ethyl benzoate and then reacting it with hydrazine to get desired hydrazide, since I can get sodium benzoate easily
(but unfortunately not methyl salicylate), but after seeing how much work is the synthesis of methyl benzoate (look UC235 video at youtube http://www.youtube.com/watch?v=XCVx759TtNQ) I'm now off.
Interesting if this will work also with ethyl acetate (very OTC!) + hydrazine to give acetic acid hydrazide and then turning it into azide with HNO2,
but the latter may be too dangerously explosive to deal with, what do you think?
[Edited on 1-4-2015 by papaya]
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by Rosco Bodine  | This scheme is something that may fit my idea about synthesis of azide in decent yield being possible using aqueous solutions. Digesting the aryl
azide with lime water should precipitate a low solubility calcium salt of the carboxylic acid and leave highly soluble calcium azide in the filtered
solution. Reaction with sodium carbonate would precipitate calcium carbonate and leave highly soluble sodium azide in solution, evaporated to obtain
crystallized sodium azide.
Could simply reacting the hydrazine product with MEK, or acetone azine, mixed with methyl salicylate, then produce the same salicylic acid hydrazide
as the product?
[Edited on 31-3-2015 by Rosco Bodine] |
Very good synthetic pathway to NaN3.
MEK azine or Propanone azine may be used as a discrete source of hydrazine (see USP 6002015 : Method of producing 1,2,4-TRIAZOLE) with water at high
temperature (170°C listed in the patent).
But there is a chance the keton may react with the phenol compound to generate para-bonded alcool and finally alkene...and further polymeric
material...
Usually it works with aldehyds like formol/ethanal (see phenol-formol thermoplastic); methylol enters easily in all ortho and para position.
HO-C6H4(CO2H) + (CH3)2C=O -??-> HO-C6H3(CO2H)-C(OH)(CH3)2 --> HO-C6H3(CO2H)-C(CH3)=CH2 + H2O
So it is maybe better to do without the hydroxy/phenol --> benzoates.
Also the presence of the phenol may be a problem at high temperature involved for the hydrolysis of the azine because as explained elsewhere phenol is
in equilibrium with a keto form what will for sure take some hydrazine. The -OH is substituable by -NH2, by -NH-NH2 and maybe -NHOH (and reversely).
See conversion of TATNB into TNPhloroglucidol by diluted NaOH and NH3 evolution....or the conversion of TNP into TNA upon heating and saturated NH3.
HO-C6H5 + NH2-NH2 --> NH2-NH-C6H5 + H2O
HO-C6H5 <--> O=C6H6
O=C6H6 + NH2-NH2 --> NH2-N=C6H6 + H2O
NH2-N=C6H6 <--> NH2-NH-C6H5
Also possible in the final product C6H5-NH-NH-C6H5 and C6H5-N=N-C6H5.
Last but not least phenol is reactive towards the acyl azide leading to an urethane:
Ar-CO-N3 + HO-Ar --> Ar-NH-CO-O-Ar + N2
[Edited on 1-4-2015 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
If the Azines could be problematic because of unwanted reaction of the ketone fragment, perhaps better to just avoid them and work with an aqueous
solution of the free hydrazine, but I don't know how good would be the reaction seeking formation of the hydrazide using a much more dilute aqueous
hydrazine. The literature describes use of hydrazine hydrate but mentions no concentration requirements other than showing a much lower yield for the
ethanol diluted mixture reacted under reflux.
The idea of microwaving hydrazine doesn't exactly fill me with enthusiasm 
Working from methyl or ethyl benzoate would avoid those complications noted for the hydroxy benzoic esters. Formation of the hydrazide reminds me of
transesterfication.
Deja Vu is strong for me about this idea and I think I may have already looked at this same idea or very similar alternative idea as a potential route
to azides some years ago in the azide thread and looking at very old literature there.
|
|
|
papaya
National Hazard
   
Posts: 615
Registered: 4-4-2013
Member Is Offline
Mood: reactive
|
|
Is there any good source of methyl or ethyl benzoates, or easier synthetic route to them? What about using ethylacetate ?
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by papaya  |
Interesting if this will work also with ethyl acetate (very OTC!) + hydrazine to give acetic acid hydrazide and then turning it into azide with HNO2,
but the latter may be too dangerously explosive to deal with, what do you think?
|
True that low molecular weight acyl azide are explosive.
CH3-CO-N3 is a liquid very prone to explosion in the concentrated state. The Curtius rearrangement must be done in an inert solvant that serves as
manyfold diluant.
Even if there is no explosion the liberation of a good volume of N2 and volatile, flamable, toxic and carcinogenic methyl isocyanate (CH3-N=C=O) is
not to neglect!
Other poly-acyl-azide are very dangerous explosive in the crystalline form: Fumaryl diazide, Malonyl diazide, Ethane-tetracarbonyl tetraazide.
As a rule the more carbon per azido group the best. High explosivity for 1/1, 2/1, 3/1.
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by Rosco Bodine  | If the Azines could be problematic because of unwanted reaction of the ketone fragment, perhaps better to just avoid them and work with an aqueous
solution of the free hydrazine, but I don't know how good would be the reaction seeking formation of the hydrazide using a much more dilute aqueous
hydrazine. The literature describes use of hydrazine hydrate but mentions no concentration requirements other than showing a much lower yield for the
ethanol diluted mixture reacted under reflux.
The idea of microwaving hydrazine doesn't exactly fill me with enthusiasm 
Working from methyl or ethyl benzoate would avoid those complications noted for the hydroxy benzoic esters. Formation of the hydrazide reminds me of
transesterfication.
Deja Vu is strong for me about this idea and I think I may have already looked at this same idea or very similar alternative idea as a potential route
to azides some years ago in the azide thread and looking at very old literature there. |
There is always the possibility to increase hydrazine concentration by making the hydrochloride...isolate it by evaporation and allow it to react with
solid Ca(OH)2 or NaOH in ethanol.
Won't you give a microwave for the science?
Microwave to Houston, do you copy, we have reached geostationnary orbit 
Yes hydrazide formation is linked to transesterification and to transamidation...
R-CO-O-R' + R"OH <--> R-CO-O-R" + R'OH
R-CO-O-R' + R"-CO-O-R"' <--> R-CO-O-R"' + R"-CO-O-R'
R-CO-NH-R' + R"-NH2 <--> R-CO-NH-R" + R'-NH2
R-CO-NH2 + NH2-NH2(l) <-=> R-CO-NH-NH2 + NH3(g)
R-CO-NH2 + H2N-OH(l) <-=> R-CO-NHOH + NH3(g)
2 R-CO-OH + NH2-CO-NH2 <-heat=> 2 R-CO-NH2 + CO2(g) + H2O
And a last but not least reaction  especially for Rosco especially for Rosco  ! !
Ar-CO-N3 + 2 NH3 --> Ar-CO-NH2 + NH4N3
The NH4N3 is said to be scarcely soluble in water (how much?), while the Ar-CO-NH2 (benzamide) is soluble to the extend of 1,35%.
But NH4N3 is quite stable, unsensitive and volatile (high vapor pressure)...what might be valuable for isolation from the mix (Under vaccuum -->
sublimation on a cold surface).
The resulting benzamide may then be reacted with hydrazine and recycled into benzoyl hydrazide, it is much less, time and energy consuming than
working via benzoic ester again  : :
R-CO-NH2 + NH2-NH2(l) <-=> R-CO-NH-NH2 + NH3(g)
The resulting benzoyl hydrazide is also not very soluble in water (how much?).
A final idea based on the above to spare one more step:
Ar-CO-N3 + 2 NH2-NH2 --> Ar-CO-NH-NH2 + NH2-NH3N3
If hydrazinium azide is more soluble than benzoyl hydrazide --> isolation via solubility.
Otherwise there is a chance hydrazinium azide is like ammonium azide, also volatile --> sublimation in the cold vaccuum.
Does someone have references for hydroxylamine azide?
HONH3N3
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by papaya  | | Is there any good source of methyl or ethyl benzoates, or easier synthetic route to them? What about using ethylacetate ? |
That is maybe a good idea!
Ar-CO2H + CH3-CO2Me -heat-> Ar-CO2Me + CH3-CO2H
Ar-CO2H + CH3-CO2Et -heat-> Ar-CO2Et + CH3-CO2H
Maybe to be in a more practical T° range butyl acetate or ethyl formate.
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Quote: Originally posted by papaya  | | Is there any good source of methyl or ethyl benzoates, or easier synthetic route to them? What about using ethylacetate ? |
About sourcing those esters or their synthesis, I have not done a thorough research but the esters appear to be easily synthesized.
Another example of the potentially useful esters would be methyl oxalate which would lead to a dihydrazide and ultimately to oxalyl diazide which
could have interest itself. I don't think that oxalyl diazide has been discussed here before and I can't find many references about it other than it
does exist. Maybe oxalyl diazide is another obscure item of interest identified for Klapotke and his group 
http://www.sigmaaldrich.com/catalog/product/aldrich/131296?l...
Oxalic Dihydrazide
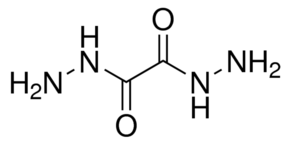
converted by reaction with nitrous acid to
Oxalic Diazide C2O2N6
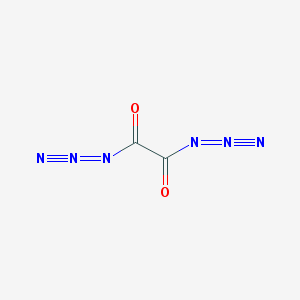
Update: The oxalic acid diazide reportedly explodes upon attempts at isolation. I knew it had to be energetic and only marginally stable. But
evidently it is only barely stable at all.
http://onlinelibrary.wiley.com/doi/10.1002/cber.19640970630/...
https://books.google.com/books?id=i0AEIrEUfg0C&pg=PA151&...
Yes there can be used ethyl acetate which leads easily to a 98% conversion to Acetic Acid Hydrazide. I am attaching a couple of ancient articles by
Curtius also which have identified this specific precursor being converted to acetyl azide, See page 10 of the pdf, journal page 463 chart last item
in article
Curtius - Hydrazine und Azide Organicher Sauren (attached)
Also see page 13 of the pdf for a second Curtius article (attached). Evidently the type reaction scheme is general.
Attached also is an article showing synthesis of the hydrazides, following a scheme that is also general.
As a matter of simplicity I think it is probably an easier more practical route to sodium azide to work from semicarbazide or another hydrazide which
can be made directly from the chlorourea process, which eliminates having to first make hydrazine and then convert an organic acid ester to a
hydrazide.
The simplest ever reported synthesis of an azide by Angeli and developed later by Hodgkinson for silver azide is sort of the "holy grail" of a simple
aqueous reaction system synthesis. However, it is a deviously fickle reaction requiring very exacting pH control, requiring a methodology never fully
disclosed. Engager said that the Russians had worked out the details of that process, and evidently the British war college has also long ago done
that same work. But there is no comprehensive reference cited or available published anywhere that I have found. Possibly the exact details about that
process for silver azide are something of a secret, for which we are missing the file. The information may reside in some memoirs as hard copy in a
reading room never digitized or published, or has been classified secret and remained classified (not for publication).
It is long overdue that some detailed description of that Hodgkinson / Angeli route to silver azide be published with full disclosure of the
particulars. Bromothymol Blue and Bromocresol Purple and Alizarin and Methyl Red indicators along with a digital pH meter with some graphing and
video coverage would be good. So long as we are wishing, why cut corners  Now
there's an experiment! Now
there's an experiment!  An oldie but a goodie revisited, remastered. An oldie but a goodie revisited, remastered.
https://www.youtube.com/watch?v=NowbmnpXYvU
<iframe sandbox width="640" height="480" src="https://www.youtube.com/embed/NowbmnpXYvU?rel=0" frameborder="0" allowfullscreen></iframe>
This thread from the discussion shift to azides should probably be split and merged with the sticky azides thread.
Attachment: Curtius - Hydrazine und Azide Organicher Sauren.pdf (381kB)
This file has been downloaded 620 times
Attachment: Curtius Journal_of_the_Switchmen_s_Union.pdf (691kB)
This file has been downloaded 532 times
Attachment: optimized production of acetylhydrazine.pdf (132kB)
This file has been downloaded 1259 times
Attachment: Benzoic Acid Hydrazide.pdf (435kB)
This file has been downloaded 961 times
Attachment: benzoic acid hydrazide from methyl benzoate and alcohol containing hydrazine.pdf (119kB)
This file has been downloaded 866 times
[Edited on 2-4-2015 by Rosco Bodine]
|
|
|
papaya
National Hazard
   
Posts: 615
Registered: 4-4-2013
Member Is Offline
Mood: reactive
|
|
Wow, Roscoe that iranian paper on acetyl-hydrazine synthesis from ethylacetate with 98% yield is fantastic, actually this is kind of pour together and
heat reaction type, interesting how to isolate the product after synthesis. Then it can be carefully converted into acetyl azide (in a very dilute
form) and then for example to ammonium azide reacting with ammonia. In azide thread I found an attached paper where it's written ammonium azide can
be "distilled" with water steam, another route for isolation.
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Hennig Brand  | Thanks for locating that. We have a nice collection of old articles now (pieces of the puzzle).
It is the first paragraph from the first article of the two that I posted above, where your article is referenced, which immediately got me very
interested at the time.
"It has been found by the previous workers that the sulphonic acid group
in either 3-sulpho- or 5-sulpho-salicylic acid can easily be substituted by
the nitro group, and if the reaction is carried further, the -COOH group is
removed and picric acid is the usual and the only product. Thus Datta
and Varma obtained picric acid by the action of nitrous gases on 5-sulphosalicylic
acid. Meldrum and Hirwe obtained 3 : 5-dinitrosalicylic acid by
controlled nitration of 5-sulphosalicylic acid."
3 : 5-dinitrosalicylic acid is the intermediate just before decarboxylation and addition of the last nitro group forming picric acid.
|
Found another one of the old articles on my other computer.
Attachment: 3-Sulfo Salicylic Acid, etc - Meldrum and Hirwe.pdf (7.8MB)
This file has been downloaded 879 times
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
The article described use of cool temperatures could have effect on the order of nitration substitution for the carboxyl so it could be different for
the example given as compared with a higher temperature and use of a solid nitrate for the nitration, under conditions where also nitrosyl sulfuric
acid is present. I think the conditions of nitration could have bearing on when the decarboxylation takes place.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Quote: Originally posted by papaya  | Wow, Roscoe that iranian paper on acetyl-hydrazine synthesis from ethylacetate with 98% yield is fantastic, actually this is kind of pour together and
heat reaction type, interesting how to isolate the product after synthesis. Then it can be carefully converted into acetyl azide (in a very dilute
form) and then for example to ammonium azide reacting with ammonia. In azide thread I found an attached paper where it's written ammonium azide can
be "distilled" with water steam, another route for isolation.
|
Yeah it seems pretty straightforward. I am still intrigued by the methyl oxalate to oxalic hydrazide to oxalic azide route because there are other
things like oxalic azide which can't be safely isolated that are still very useful intermediates in synthesis where they are immediately converted to
something else and never isolated. I was thinking if the oxalic azide is produced in good yield then hydrolysis by calcium hydroxide would
precipitate low soluble calcium oxalate and leave highly soluble calcium azide in solution, which could be easily converted to calcium carbonate also
precipitated by sodium carbonate, leaving a sodium azide solution. It might even be possible as a one pot reaction.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by Rosco Bodine  | Quote: Originally posted by papaya  | Wow, Roscoe that iranian paper on acetyl-hydrazine synthesis from ethylacetate with 98% yield is fantastic, actually this is kind of pour together and
heat reaction type, interesting how to isolate the product after synthesis. Then it can be carefully converted into acetyl azide (in a very dilute
form) and then for example to ammonium azide reacting with ammonia. In azide thread I found an attached paper where it's written ammonium azide can
be "distilled" with water steam, another route for isolation.
|
Yeah it seems pretty straightforward. I am still intrigued by the methyl oxalate to oxalic hydrazide to oxalic azide route because there are other
things like oxalic azide which can't be safely isolated that are still very useful intermediates in synthesis where they are immediately converted to
something else and never isolated. I was thinking if the oxalic azide is produced in good yield then hydrolysis by calcium hydroxide would
precipitate low soluble calcium oxalate and leave highly soluble calcium azide in solution, which could be easily converted to calcium carbonate also
precipitated by sodium carbonate, leaving a sodium azide solution. It might even be possible as a one pot reaction. |
@Rosco,
Very nice idea fro Ca(N3)2 and Ca oxalate...
If oxalyl diazide is too critical for practical use...maybe tartric acid diazide is an alternative.
N3-CO-CHOH-CHOH-CO-N3 --> Ca(N3)2 and Ca tartrate
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Another thought I had is that by modifying the diazotization of the oxalic dihydrazide perhaps using calcium nitrite directly or maybe with pH
adjusted by acetic acid or some other acid, that formation of oxalic diazide may be avoided and calcium azide hopefully would form directly, which
would remain in solution with the byproduct calcium oxalate precipitated.
An unknown exists that the same sensitivity to pH and solubility factors as is seen for the Angeli / Hodgkinson reaction for producing silver azide
may likewise occur for these similar reactions. It may be found that these reactions are technically possible but are are sensitive about reaction pH
conditions to the point that practicality suffers.
A diazotization by an organic nitrite might work better also, and will proceed at a basic pH which may even be required to prevent the escape of
volatile HN3.
Further treatment with sodium carbonate or ammonium carbonate or potassium carbonate would then precipitate the soluble calcium value of the calcium
azide as calcium carbonate, and the filtered solution evaporated to obtain the desired azide.
This same general scheme might work for some other cases, like tartaric acid hydrazide or other hydrazides.
[Edited on 8-4-2015 by Rosco Bodine]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
some old references and a new article
Attached are some old references about picric acid and picrates
Attachment: Pages from The_Chemical_Gazette 1859 pg 1 to 8.pdf (506kB)
This file has been downloaded 432 times
Attachment: Pages from The_American_Journal_of_Science pg 379 to 385 M. Carey Lea picric acid and picrates.pdf (671kB)
This file has been downloaded 392 times
Attachment: Pages from The_American_Journal_of_Science pg 78 to 86 M. Carey Lea ammonium metallic picrates.pdf (829kB)
This file has been downloaded 419 times
Additionally is a recent and new article about a one step nitration of phenol in DMSO using HNO3, which may be more general and possibly applicable to
nitration of other substances than phenol.
Attachment: Picric Acid via nitration of phenol in DMSO.pdf (398kB)
This file has been downloaded 1475 times
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
The Metallic Picrates
Here attached is a more legible scan of an article posted earlier.
Attachment: The Metallic Picrates - Silberrad and Phillips.pdf (682kB)
This file has been downloaded 434 times
|
|
|
joseph6355
Hazard to Others
  
Posts: 144
Registered: 23-8-2017
Member Is Offline
Mood: Nitrated
|
|
Is recrystallizing picric acid from filtered tap water too stupid?
I mean, I'm aware that picrates will be formed, but will them be in considerable amounts enough to make the final material dangerous?
I will be casting it, btw.
Distilling water has become tedious, and buying it is a pain in the ***, because its heavy.
My reagents are technical grade, and my ASA is ACS grade. Is recrystallization a must considering that I will cast it?
[Edited on 18/4/18 by joseph6355]
Oh, hello!  |
|
|
XeonTheMGPony
International Hazard
    
Posts: 1636
Registered: 5-1-2016
Member Is Offline
Mood: No Mood
|
|
what do you consider acceptable purity?
Clean pure product behaves reliable in predictable manner! why I distill store bought acetone and other reagents.
|
|
|
PhenethylamineMachine
Hazard to Others
  
Posts: 110
Registered: 22-3-2018
Member Is Offline
Mood: No Mood
|
|
I have been invested in a related project. This post is marking the topic so I can monitor it.
|
|
|
| Pages:
1
..
24
25
26
27
28
..
31 |