| Pages:
1
..
17
18
19
20
21
..
31 |
The WiZard is In
International Hazard
    
Posts: 1617
Registered: 3-4-2010
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by quicksilver  |
Re: those lead blocks, I wish I could post a picture because the one that I had looked like it fit a detonator. but I've read about ones that could be
used for free materiel w/ tamping so the hole HAD to be bigger than 6mm.
I'm fairly sure a really accurate example could be home-made for just a few dollars.
[Edited on 30-7-2010 by quicksilver] |
Sure, however, if one wanted to publish their results the lead
block would have to meet the current standard.
Occam would have approved of this :—
Drop and friction pendulum tests were designed to replace the old English Dupre tests. (Dr. August Dupre 1835-1908).
"A small quality of the explosive is spread on a large stone table and is struck a glancing blow
with a mallet; the explosive is thus subject to the combined effect of shock and friction. Use is made of two
sorts of mallets, one of rawhide and the other of beech wood, similar to those used by wood carvers. The
rawhide mallet weighs about 12 ounces and the wooden one about 11 1/2 ounces. The rawhide mallet is
more effective, especially with chlorate explosives. If the sample explodes in this test it is repeated using a
broomstick instead of the mallets. The broomstick is held on the stone at an angle of about 60o and a blow
is struck with its end, taking care that the movement of the stick is in the direction of its axis. If an
explosion is obtained in this way the test is repeated, the explosive being placed on a hard wooden table
instead of the stone one and, finally, if necessary, on a soft wooden table. If an explosive other than those
used for caps, detonators, etc., explodes even partly on softwood it is considered too sensitive for its use to
be authorized. Needless to say, the mallets, broomsticks, and tables must be clean." (Physical testing of
explosives. Bureau of Mines Bulletin 346. 1931)
See also :—
S. P Howell
Sensitiveness of Explosives to Frictional Impact
US Bureau of Mines Bulletin Technical Paper 234. 1919
http://tinyurl.com/27rh885
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Rosco, what would be the consequence of not doing the addition slowly into the Lead Nitrate solution? I probably messed up the lab out of impatience
and not fully understanding the process. I made the addition very quickly then heated for fifteen minutes or so to around 80C. Stirring was not what
it should have been either. I didn't look at the product under a microscope but what I had looked more like a dense amorphous powder to the naked eye.
It is very sensitive to flame(sort of flashes like Mercury Fulminate when lit in a tiny pile in the open). It is quite insensitive to percussion.
Here is a picture of about 12g of the material I made, I don't know if you can tell much by the picture.

|
|
|
The WiZard is In
International Hazard
    
Posts: 1617
Registered: 3-4-2010
Member Is Offline
Mood: No Mood
|
|
Trauzel on steroids
RELATIVE STRENGTH OF EXPLOSIVES
For finding the relative disruptive power of different explosives,
and thus enable the officers to apportion the amount of charge to
be used in testing, a modified form of the Trauzel lead-block
method was adopted Although this method has since been
superseded it will be of interest to briefly describe the process and
some of the experiments. The blocks in use at Woolwich were of
cylindrical shape 12 inches high bv 12 inches diameter, with an
axial hole drilled to a depth of 7 inches having a capacity of 5 fluid
ounces. In the early experiments the blocks were frequently
recast, but some results having brought suspicion upon the
accuracy of the tests it was decided to use only "virgin" lead blocks
for each test. When 1 ounce of No. 1 dynamite was fired in the
cavity, with stemming, the expansion of the cavity would be about
30 fluid ounces, as compared with an expansion of only 3 ounces
from the same weight of gunpowder, which made dynamite
appear ten times stronger than gunpowder. Experiments in other
directions having proved that the real proportion of power was
about 3 to 1, an examination of the block method led to the
adoption of placing a considerable weight over the charge to be
fired when gunpowder was being tested. Another of the lead
blocks, weighing 5 hundredweight, answered the purpose. This
placed over the orifice thoroughly confined the charge, and
brought out approximately fair results. With a high explosive the
effect would be brought out by the tamping of dry sand. In this
way were obtained the following figures, showing the relative
strength of different explosives by equal charges, and the weight
of equivalent charge required for the same amount of work done,
taking dynamite No. 1 at 100:
http://tinyurl.com/25wdp7m
|
|
|
The WiZard is In
International Hazard
    
Posts: 1617
Registered: 3-4-2010
Member Is Offline
Mood: No Mood
|
|
http://tinyurl.com/25wdp7m
If'n you DL the 780 page PDF so you can print it out - this is PDF pages 340-356.
Has good photos (2) of the Bureau of Mines Friction Pendulum.
|
|
|
The WiZard is In
International Hazard
    
Posts: 1617
Registered: 3-4-2010
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by quicksilver  |
Re: those lead blocks, I wish I could post a picture because the one
that I had looked like it fit a detonator. but I've read about ones
that could be used for free materiel w/ tamping so the hole HAD to
be bigger than 6mm.
I'm fairly sure a really accurate example could be home-made for
just a few dollars. |
Try - http://tinyurl.com/3y9x52m
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Quote: Originally posted by Hennig Brand  | Rosco, what would be the consequence of not doing the addition slowly into the Lead Nitrate solution? I probably messed up the lab out of impatience
and not fully understanding the process. I made the addition very quickly then heated for fifteen minutes or so to around 80C. Stirring was not what
it should have been either. I didn't look at the product under a microscope but what I had looked more like a dense amorphous powder to the naked eye.
It is very sensitive to flame(sort of flashes like Mercury Fulminate when lit in a tiny pile in the open). It is quite insensitive to percussion.
Here is a picture of about 12g of the material I made, I don't know if you can tell much by the picture. |
You may have made what Legard thinks is basic lead picrate but believe me that is not basic lead picrate, so then you may safely assume that order of
addition, temperature, dilution, and reaction rate are important variables in real actual chemistry done by real actual chemists and not just fiction
novels having some dreamt up reaction scenarios written by assholes who are pretenders. If you want to make basic lead picrate then go by the method
of the Friederich patent. If you want crap, follow the Legard wetdream and mental masturbation guidebook for chemist wannabees. (caveat emptor -
translation provided)
What the bright yellow material gotten from the reverse order of addition could possibly be is a hydrated intermediate and/or possibly contaminated
double salt. It may be possible to accomplish its conversion by long stirring it in very hot almost boiling water. The use of very hot water is in
part necessary for decomposition (dehydration) of a hydrated intermediate which leads to the end product.
All of these basic lead picrate derivatives are made by a dropwise very slow addition into an excess of dilute and hot (95C works well) and vigorously
well stirred lead nitrate solution, an hour is good as a kind of rule of thumb for the addition. By vigorous stirring that is meant "suspension" of
the solids, not islands of solids accumulating on the bottom, but everything circulating in a vortex of liquid. If it takes a magnetic stirbar on the
bottom and a propellor stirrer above or a prop making an orbital sweep, then that's what it takes. It isn't just mix A+B any old way and get C, but
is a precise process. The basic lead picrate is the parent compound, and that can be gotten first and then gradually redissolved and reacted
subsequently to form daughter compounds. That is the variation which I prefer and is allowable as a process refinement, but is more of a long way
around than any shortcut. Actually there probably is no shortcut process for such low solubility reactants where the reaction has to be pushed with
heat and dilution and efficient stirring. It isn't complicated really, it is just that the reaction conditions are quite specific.
[Edited on 31-7-2010 by Rosco Bodine]
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
I'll give the (long way)/(proper way) a run through and see if that works better. It most likely will, I am just a little unsure as to why. One thing
I did learn is you don't think very highly of Legard, and I guess I can see why. I think his Lead Picrate synthesis is not that far off though. I am
sure I messed it up. I will do it properly and get back to you.
Oh, I found a little immersion heater. I can control this with a light dimmer and use with an oil bath, together with my magnetic stirrer, to heat and
stir. Should work well when making TNP as well.
BTW thanks for the nice patents.
edit:
Thanks for elaborating in you last post, appreciate it.
[Edited on 30-7-2010 by Hennig Brand]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
No problem. You try it the other way around and let your eyes tell you that something different is occurring and something very different in
appearance
is also being the end product. Chemistry can be a real bitch about specific
conditions. And how well do I know that a lot of folks would argue about details which they may think make no difference and are of no consequence,
when
there are subtleties involved in the reaction that are simply not recognized.
But as they say, the proof is in the pudding, or crystals in our case. You'll see the difference. Go very slowly and watch for the color shift and
the change thresholds and you will see that bright yellow shift to orange brown right before your eyes. I promise you can visually discern the
transition as a distinct change in appearance and viscosity also. The needle like felt fiber like bright yellow initial precipitate will transition
to a darker orange brown compact crystalline material having much denser and reflective appearance.
It will be more like a dense fine sparkling orange brown sand than a fluffy yellow amorphous garbage.
[Edited on 30-7-2010 by Rosco Bodine]
|
|
|
quicksilver
International Hazard
    
Posts: 1820
Registered: 7-9-2005
Location: Inches from the keyboard....
Member Is Offline
Mood: ~-=SWINGS=-~
|
|
@ Wizard:
Excellent hits: Thanks. I honestly appreciate it when you find material correlated to questions (because generally that means I don't have the
resource or it's too damn buried).
I have bought books from Albris ( the book seller in the link) and was really happy with what they had and the way they described condition. I an
going to see what they have from Vannoy Hartrog Manning, etc. IF you're ever going to buy, I recommend Albris. They are not the cheapest but I always
get high quality from them (bindings aren't shit, etc) and they had the PATR as individual volumes from various libraries. - NOT as one $1000 set.
To reiterate, there WAS a time when USBoM material was available for D/L. It was a long time back. I think you would go to the US Dept. of the
Interior & do a search for US Bureau of Mines and they had a page that told the story and how it was defunct but had a "search" component that
would lead you to another page of the papers in numerical order which you could access. Back then I don't think they were in PDF but you could get
them by doing a "print page" and having some manner of PDF printing mechanism. The US Patent Office has a link to use a TIFF printing utility which is
not that much of a hassle to put in to a PDF. The utility is called ALTERNATIFF.exe if you ever want to snag unique patents from the gov web page.
It's a plug-in for a browser made to work with their web site to view print or save the patent as it was filed.
I am going to try the Bureau of Land Management; as they may have taken the documentation from the USDoI in so far as mining.
If I find the collection, I'll post the link - If you should ever find anything please let me know!
[Edited on 30-7-2010 by quicksilver]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
I am not a "professional chemist" neither am I a pilot but I can fly an airplane.
You don't have to be a carpenter to hit a nail on the head, you just need the right tool for the job and to pay attention to get it done correctly.
I have contempt for the Legard books because they are rife with error to the point of unacceptability and exposing of the fact that the author is no
authority regarding the material which is collected research of others, neither understood nor confirmed by the author. Such literature is
unenlightening.
[Edited on 30-7-2010 by Rosco Bodine]
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
I found with the Picric Acid synthesis, as long as I had excellent magnetic stirring, that alot of the other things were much less important. A lot of
the "recipes" floating around don't even discuss the need for good stirring. I would say it is even more important than temperature control maybe(up
to a point).
I have probably read too much fringe literature garbage, which is likely why I am always suspicious of synthesis details. Usually if I experiment with
a particular supstance long enough I develop an understanding of why certain things are done or not done in a synthesis. My biggest fault most likely
is always trying to find an easier way out
I am going to give it another shot, tommorrow maybe.
I deleted that last edit from my last post as you and Quicksilver had already posted, and it didn't seem to fit anymore. Yeah, I guess one needn't be
a proffessional to be good at something.
Here is the deleted edit, don't know if it adds anything, but hey.
One thing I would like to add. Now I am not a proffessional Chemist or anything, but it seems to me that sometimes there are very elaborate procedures
that are designed to get maximum efficiency/yeild, when a much simpler approach would have gotten you 95% of the way there(which most times is good
enough for me). Other times(and this is starting to look like one of those times), cutting corners can really cost you. I believe Einsten was often
noted along with quite a few other famous scientists for saying that the established academia quite often made things much more complicated than what
really need be, and many folks would be able to understand much more if it were not for this(or at least it would be easier). On the other hand, we
can't dumb everything down to the level of every scienctifically challenged individual. We are seeing the consequences of that now in many schools and
society. This is all just my opinion, not being critical of advice given at all.
[Edited on 30-7-2010 by Hennig Brand]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Objection your honor, counsel for the stirrer is arguing facts not in evidence and contradicted by the thermal realities applicable to the reaction
conditions 
Stirring is important for keeping the reaction temperature evenly distributed along with the reactants in the mixture, however heating is generally
required in aromatic nitrations where higher nitrations are being accomplished, and the reaction rate is greatly slowed or incomplete absent that
heating. In large reaction masses conservation of the heat of reaction itself may be sufficient, but for smaller scale batches where radiational
cooling causes the reaction to be deficient in temperature to maintain the reaction to endpoint, then supplemental heating is required. You got to
work with the thermodynamic that is applicable, whether it involves supplemental heating or cooling and that varies with batch size.
[Edited on 30-7-2010 by Rosco Bodine]
|
|
|
The WiZard is In
International Hazard
    
Posts: 1617
Registered: 3-4-2010
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by quicksilver  |
I have bought books from Albris ( the book seller in the link) and was really happy with what they had and the way they described condition. I an
going to see what they have from Vannoy Hartrog Manning, etc. IF you're ever going to buy, I recommend Albris. They are not the cheapest but I always
get high quality from them (bindings aren't shit, etc) and they had the PATR as individual volumes from various libraries. - NOT as one $1000 set.
|
I never recommend Alibris anymore, I mentioned it on the
Venom mailing list several years ago, and someone with
a lot larger excheque then mine apparently bought up everything
including a $600 book in Latin.
You can DL PATR 2700 in theory from dtic.mil, there are a number
of PDF copies for sale on the cheap, however, both are image
copies and are not searchable.
I am finding more Bureau of Mines stuff @ google.com/books
and have just found Bichel's historical New Methods of Testing
Explosives, 1905.
|
|
|
The WiZard is In
International Hazard
    
Posts: 1617
Registered: 3-4-2010
Member Is Offline
Mood: No Mood
|
|
Google.com/patents are already in PDF format.
|
|
|
The WiZard is In
International Hazard
    
Posts: 1617
Registered: 3-4-2010
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by quicksilver  |
US1478429 makes reference to nitrated cresol as a building block. Cresol is (IMO) much easier to nitrate than phenol but very difficult to obtain,
which is a shame because there are quite a few things that employ it. The reagent-grade material is quite expensive & unnecessary, yet I have not
found a source for anything but high-end cresol. I would really appreciate it if anyone who does know more about where to obtain same would post their
experiences. {TNC is very close to TMP in a variety of tests. |
You have a chemical engineering problem. Cpt. Colver notes that
when you nitrate crude cresol ... as soon as the the dinitro
stage has been passed the dinitro products of o- and p-cresol
are oxidized, and at the conclusion of the process only trinito
meta-crysol remains.....
.... commercial cresol gives only a yield of 90-100% in comparison
with the theoretical value of 220%, pure meta-cresol gives a
yield of 150% on trinitometacrysol.
Me thinks you will find buying a tank car load of the commerical
product vs a pound or two. Try the Chemical Week Buyers Guide.
Though getting someone to sell it to you........!
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Well you certainly have a way with words Rosco. I have to be more careful with mine when I am around you, I can see that
Certain temperatures are very important as it controls what will or will not happen in an aromatic nitration, but isn't the stirring more responsible
for how fast it happens? I just recently realized by not having mechanical stirring for the last stage of TNP nitration that I was not getting nearly
as complete a nitration in the time taken(meaning I wasn't getting as good a nitration practically speaking, in effect). In small scale unless using
reflux the HNO3 can be being distilled away mighty fast as well. Does this seem more or less right, not really contradicting you I think.
Just did first year general Chemistry for Engineers this year. I did alright, but to tell the truth untill I get my hands dirty a little bit the
theory only goes so far. Its one thing to regurgitate some theory and pass a test(well there is some understanding), but another to really apply
things in a real situation. I always figured some of the career science types lived in the abstract better than I did. My personal view is you need
both to do things right. Sorry for the rant.
Just did the Basic Lead Picrate synthesis again, couldn't wait until tommorrow.
The only thing I did a little different from the basic method in the patent 1478429 for Basic Lead Picrate was keep the temperature around
40-50C(because it was inconvenient to do otherwise tonight). I also did the addition in about an hour as you suggested, instead of the 1/2 hour the
patent suggested.
To tell the truth I was a little disapointed at first. It still looks orange even after 2-3hours in the oven, not red. The crystal structure is very
fine, but that is what the patent said would happen with reduced temperature. I next tried a small burn test, less than a tenth of a gram for sure. It
was much more energetic, lit in the open it made quite a loud pop(sort of like lighting a little pile of HMTD only much louder, a whump I guess). I
don't know what the difference is but you seemed to know what your were talking about. Things had to happen gradually and in sequence I guess. The
before picture must be a mix of something, with LP in it.
One question, how necessary is using a perfectly pure sample of Picric Acid? Maybe a silly question, but this could never be considered a sort of
purification of sorts(going form TNP to Picrate)?
[Edited on 31-7-2010 by Hennig Brand]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Near boiling temperature is your friend here, believe me. Hydrates are unstable in the hot water so what you get is going to be the lowest hydrate or
anhydrous product if you precipitate it from a hot solution. On some reactions a cycling of temperature from lower to higher is necessary because the
hydrated expanded matrix is an essential precursor for the thermally induced destabilization and collapse and dehydration to the crystalline form, so
it may have to go in a two stage process for some materials.
Just for grinning curiosity try increasing the temperature to just below bp and increase the dilution by 50% for the Friederich patent process and see
what you get. Orange would be more like it on the color too, definitely not yellow.
The crystals should be non caking free flowing like hourglass sand. They should pour right off the filter paper when dry
with almost no fines or dust. I just checked my old lab notes on the Friederich patent process and found the product described as reddish orange
brown in color. Yield by Friederich method 8.5 grams, 94% of theory.
When there is a simple reaction mixture where everything is in solution, it is a different reaction system where the entirety of the reactants are in
contact and the behavior of such reactions tends to be linear and predictable.
Stirring can increase the reaction rate for suspensions by exposing the particles to fresh reactants and sweeping away spent reactants and products in
the reaction zone matrix. When you have a suspension of materials which are reacting in a zone between the particles or on the surfaces of the
particles, then algebra has arrived, because you actually have a layered reaction system where there are different but interactive processes occurring
simultaneously in the larger reaction mixture
which is a system, but which is the sum of subsystem reaction processes in the zones between and/or on the surfaces of the particles. The chemical
and thermal dynamics can become complex especially with increasing scale, but you can develop an ability to "read" a reaction and interpret the
general dynamic involved after observing and analyzing your experiments. Keep notes and take readings and note them and then your process logs and
end results give you answers or clues that can move things in that direction. There really is some art involved in fine tuning of chemical processes.
The precision is very exacting for some reactions, and it can be tedious sometimes to duplicate the exacting conditions even for ones own
experiments, unless careful and complete notes are kept. A digital recorder can help if you need to dictate notes which will talk yourself through
the same steps later.
Regarding the purity of reagents, it is not something I have really evaluated for significance in these reactions. I use what is probably 99.9% or
better, reasonably pure materials generally, just to eliminate variables, but it may not be necessary.
[Edited on 31-7-2010 by Rosco Bodine]
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Thanks for the good descriptions of the process.
On leaving the freshly made Basic Lead Picrate out over night, I find that this morning it has less vigor. It was hot and very dry from the oven, it
was very snappy stuff right from the oven. I used the exact same quantities of reactants as in the patent, 4.6g TNP to start, and my yeild was~6.8g of
Basic Lead Picrate. It does have a crystaline structure but a microscope at 40X(lowest magnification) was needed to clearly see it. I did notice that
when dry this time it broke up to a powder very easily, the last time it stuck together badly. I guess I need to try and make bigger crystals.
BTW still very insensitive to percussion, and mine looks close to a mustard yellow(a darker more orange mustard though).
My TNP purity may be the problem, I have been saving my best stuff for compound caps.
Lead Nitrate was made with waste acid from RDX synthesis(takes a while with only 20-30%HNO3, but waste not want not). Heat helps a lot.
Here is a picture of my yeild

[Edited on 31-7-2010 by Hennig Brand]
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Here is a couple of improvised microscope pictures. It is crap quality, but maybe you can see the crystals sort-of. Regular microscope just held the
camera up to it. Also 40X seems to be the lowest power.
 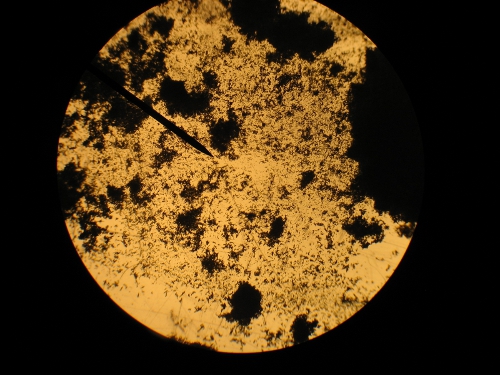
[Edited on 31-7-2010 by Hennig Brand]
|
|
|
quicksilver
International Hazard
    
Posts: 1820
Registered: 7-9-2005
Location: Inches from the keyboard....
Member Is Offline
Mood: ~-=SWINGS=-~
|
|
Basic Lead Picrate (crude picture using cellphone).
Although out of focus, note color in relationship to yellow legal pad paper.
Material is highly reactive, responding to heat & impact with substantial energy. Microscopic examination @ 100x revels rough crystalline elements
of undetermined shape coloration is rich throughout the crystal.

[Edited on 31-7-2010 by quicksilver]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
@quicksilver That rust like color is more like it 
@Hennig Brand You got something different from the expected result.
Something I don't understand is the drying in an oven you describe.
The product should be absolutely non hygroscopic and dry completely crisp
right on the filter, fold the filter and pour it off like sand. A few hours air drying
and it is done if you have sure enough, honest injun, bona fide genuine,
basic lead picrate.
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Nice looking stuff Quicksilver, wish mine looked like that.
I finally broke down and used some of my TNP that was saved for detonators. Using impure TNP is not a plan I would judge now. I also kept the
temperature up to around 75-80C through the whole addition. I did get a darker product but not nearly as dark as yours Quicksilver, I will attach a
picture. Also got an increased yield(~7.5g). It does glisten nice now in the light, but still small crystals. The last thing I could be doing
wrong(that I know of) and its a little stupid really, when I made my Pb(NO3)2, I heated untill it was almost completely dry, but not quite. There is
likely a bit of residual HNO3 in that liquid. Is neutral Lead Picrate a lighter color than Basic Lead Picrate? Could one of you tell me a better way
to deal with my Lead Nitrate? Thanks for you patience.
BTW, this stuff is much,much easier to set of with a hammer blow.
edit:
Just read from another thread, seems I should have redissolved my Pb(NO3)2 in water, and boiled to dryness again to get rid of most of the remaining
acid. The acid can't be helping much.
Come to think of it I did see a little bright yellow flash of color right when a drop of Sodium Picrate solution hit the (extra)acidic Pb(NO3)2
solution. Sodium Picrate solution at least partially turning to TNP solution perhaps.

[Edited on 31-7-2010 by Hennig Brand]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Neutral lead picrate is bright yellow transparent crystals. The monohydrate and the anhydrous form look identical.
Basic lead picrate is opaque rust colored crystals, much finer mesh microcrystalline form, a dense microcrystalline powder.
What the scrambled egg colored stuff is I don't know, maybe a mixture of lower nitrophenolate derivatives is a contaminate....who knows ? Could be a
mixture of the normal hydrate and the basic salt.
The yield is off too, so something is definitely haywire, precursors, process or both.
I noticed above you are talking about recrystalizing lead nitrate with boiling to dryness, do not do it that way as you will hydrolyze some of the
lead nitrate. Just cool down a hot saturated solution and harvest some crystals and air dry them. You need good accuracy on your weights and good
purity on your precursors, and good parameters on your process and then it will work. The process is pH sensitive so if you are not precise then the
result will tell about it.
[Edited on 31-7-2010 by Rosco Bodine]
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
I won't bother you with my messed up Picrate lab anymore for now.
My yield being off could easily be caused by my having to estimate a dry weight of Pb(NO3)2 from a damp weight. I'll get back to you when I have done
something better with my Lead Nitrate. Dryer and less acidic would be good.
I was not boiling to dryness originally as I was worried about damaging the Lead Nitrate, good to know. I guess redissolving in water would be ok,
heat, then air dry the last moisture away. Its kinda damp in this part of the world alot of the time, making air drying difficult.
I guess even the simple Picrate stuff can be messed up pretty bad, if your not carefull.
[Edited on 1-8-2010 by Hennig Brand]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Don't be discouraged. I measure using a centigram quad beam for accuracy to milligrams on these. The Friederich method can actually be fine tuned to
a quantitative yield if it is done carefully with stronger solutions at near boiling. Higher temperature would help. I typically do these reactions
at 95-98C.
For air drying stuff a crock pot set on a lowest heat and left open, or a sheet of styrofoam or stack of newspapers with a heating pad and a glass
casserole tray works fine. Even the low heat from a reading lamp over a dish will do the job.
[Edited on 1-8-2010 by Rosco Bodine]
|
|
|
| Pages:
1
..
17
18
19
20
21
..
31 |