| Pages:
1
..
7
8
9
10 |
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Yeah I also have a couple of references requested to add to the database we have already. There are quite a few one page communications in the
journals about small scale H2S generators using various schemes convenient for occasional experiments where H2S is needed mainly for analytical
procedures not requiring large amounts of H2S as would be used in synthesis. The scale of interest to us is intermediate so it may be adaptable from
either scenario of larger or smaller scale methods. There are likely several different valid approaches to making H2S or derivatives in intermediate
scale quantities using generally commonly available materials so the choice may come down to using what materials are most convenient. This topic and
the associated DDNP should probably achieve sticky status as it reaches maturity.
The article by Hodgson and Ward did not identify DDNP specifically as the material they used for the analytical determination of yield for their
synthesis of picramic acid. But from the H&W description it would seem that their yield calculations for picramic acid were ultimately based upon
the amount of DDNP resulting from diazotization, with their presuming the yield of DDNP would be quantitative and therefore mirror the yield of the
precursor picramic acid. If that interpretation is correct then there is some distinction lost between this picramic acid thread and the DDNP thread,
which is a small distinction anyway like part A and part B of an overall synthesis with part A being the precursor material picramic acid for DDNP
that is the tongue in cheek "yield determination" part B.
There is no indication that in the alternative that H&W were investigating the effectiveness of a red hair dye, but what they were doing is making
DDNP.
[Edited on 15-4-2014 by Rosco Bodine]
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
I did notice a while back that the yield determination from the Hodgson and Ward article involved titration of sodium picramate by conversion to DDNP.
They most likely were focused on DDNP, and the article was just discussing production of the intermediate (sodium picramate).
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
I have been looking at the various schemes for H2S generators to see what can be done as the most easily improvised method using over the counter
materials. It is something I mentioned earlier in the thread that when the "dry method" type of polysulfides mixture is acidified that H2S will be
produced but free sulfur will also be a byproduct.
So using a polysulfides mixture is not as efficient as using ferrous sulfide, but the economy is probably comparable since things like hydrated lime,
washing soda or baking soda, and garden sulfur are cheap raw materials. There have been patented commercial process reaction schemes for producing
what are "lime-sulfur" solutions that contain 27% soluble sulfur in various forms where the process is run under mild conditions, and I haven't
finished my calculations on what part of that 27% soluble sulfur would actually convert to H2S on acidifying and what percentage would simply be
precipitated as free sulfur. A possible scheme of interest is one where the polysulfides solution used is an equimolar mixture of sodium and calcium
polysulfides. It would require some experiments to sort out what is the optimal mixture. What I have been thinking is it is possible that similarly
as the mixture of iron filings and sulfur will react in the presence of H2O without it being necessary for igniting the mixture and performing the
reaction at high temperature, that possibly a similar reaction may occur in a mixture of sodium bicarbonate and hydrated lime and sulfur mixed with
water in a measured restricted quantity, with a similar reaction proceeding as when the dry method of mixing NaOH and S is done with minimal H2O just
suffficient for conducting the reaction where the mixture becomes liquified from the exotherm. With NaHCO3 plus Ca(OH)2 the NaOH should form in situ
and then react with the S and if the quantity of H2O used is correct, then the liquified Na polysulfides mixture resulting should be able to be
decanted from the insoluble CaCO3 byproduct. This Na polysulfides liquid should produce H2S when it is acidified. Or it may serve better to use
this Na polysulfides as a source of soluble sulfur residing in its polyulfide component to react with additional Ca(OH)2 which will ( I think ) tend
to form CaS by reaction with the sodium polysulfide values that are only soluble sulfur, converting them to compound sulfur as CaS which increases the
proportion of sulfides to what is convertible to H2S when acidified. Ca(OH)2 should react differently in a two stage reaction so that the soluble
sulfur values convertible to H2S upon acidification is doubled to tripled ( if what I am thinking is correct ) above what would occur using only the
"dry method" which is really a "wet method" only having carefully limited H2O to keep the reaction mixture highly concentrated, based upon only use of
the Na polysulfides mixture. The CaS requires only one mole of Ca in relation to each S whereas the Na requires 2 moles Na to form the normal sulfide
Na2S in the same scheme. So for an equimolar mixture of Na2S and CaS formed by the scheme described, I am thinking it could be a cheaper and more
efficient precursor for production of H2S upon acidification. I have thought that once a scheme modeling the manipulations is worked out using
smaller test quantities in sample bottles or flasks that the method could be scaled up easily to whatever scale is needed. Of course if NaOH is
available easily, the first reaction could be changed to accomodate and there would be no CaCO3 byproduct.
The Na and Ca indefinite sulfides mixture could also serve directly as a kind of enhanced Zinin reagent which could be used as is for a reducing
agent, apart from being useful as a source for H2S in a generator scheme.
With regards to the high temperature generation of H2S from the thermal cracking of hydrocarbons like vaseline or paraffin, I found a reference also
that a heated mixture of pine rosin and turpentine 50/50 with sulfur reportedly freely evolves H2S and is a convenient source. What is the
temperature required and if it is high enough to cause sublimation of sulfur as occurs with the petroleum hydrocarbons is not known. I suspect that
there are hydrocarbons that will easily crack at lower temperatures than petroleum and produce H2S but I don't know what materials may be useful in
that regard. Finding a combination or single material that reacts easily at gentle heat would be a winner for that method, and would probably be the
easiest way to go. Even the vaseline and sulfur method has simplicity to commend it, but requires some accommdation for the sublimed sulfur which can
complicate things. There it may be useful to have a dual output which
serves to trap sublimed sulfur, and cycle the output flow alternating through one trap or the other, heating the sulfur condensing trap that is idle
to remelt trapped sulfur it contains and return it back into the heated mixture. When that substantially cleared trap has cooled again, then the H2S
flow is diverted through it, while heating is applied to clear its parallel trap now encrusted with sublimed sulfur, with the cycle being repeated in
alternating fashion, so that a recently cleared sulfur trap is in use continually. Such a scheme would allow for the "cracking reactor" producing H2S
to run for hours if necessary without becoming plugged with sublimed sulfur in the output tube. Using a single larger tube
maybe as a coil of a few turns of 5/8 ID copper could be simpler and remain unobstructed through the duration of any single batch run. And then it
could be cleared before the next use by simply heating it to above the mp of the sulfur it has trapped so that it would flow out of the coil and
return back into the reactor. Anyway these are two ideas for how a scaleup could be done to make use of the thermal cracking scheme for H2S
generation.
[Edited on 17-4-2014 by Rosco Bodine]
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Hydrogen sulfide reacts with copper and produces corrosion products. I didn't look into it in much depth to see how much of a problem it would be. I
went with steel, since steel is resistant to hydrogen sulfide and cheaper too.
One of the attachments you provided a few posts back had a diagram of a lab scale hydrogen sulfide generation system, with inline bulbs designed to
trap the sublimed sulfur. When I saw it I thought it looked like a simple solution to one of the only flaws with the heavy hydrocarbon and sulfur,
hydrogen sulfide generation method. I think what I am going to do is use a large diameter steel U-tube to attached the generator to another vessel and
from that second vessel lead the nearly sulfur free hydrogen sulfide off through the small diameter tube I am currently using to the bubbler. The
second vessel (deposition vessel) wouldn't need to be nearly as large as the generator vessel. This is a very simple solution that I think would work.
As far as wasting sulfur, it is quite cheap as you have pointed out. Actually, most of the deposited sulfur could likely be collected from the
deposition vessel and used again. The large diameter U-tube wouldn't plug nearly as quickly and could be cleaned out between uses much more easily
than a small diameter tube.
[Edited on 17-4-2014 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Stainless is passivated by H2S according to what I understood from a brief description I read in an article and I'll try to dig up that reference
again.
Edit: Here it is, passivation of stainless steel by H2S
http://books.google.com/books?id=Rlq-812ED10C&pg=PA33&am...
I have on hand some malleable stainless tubing that is used similarly as soft copper. I think I have a couple of 2 liter wide flange inconel reaction
kettles with heating mantles in storage somewhere, too.  Now where did I put
my inconel resin kettles? or maybe they are 316, I forget Now where did I put
my inconel resin kettles? or maybe they are 316, I forget  And hey if inconel or
316 won't do it then grade 1 titanium I have on the shelf too. I'm kidding a little bit here. Actually copper should work fine, and even glass or
ceramic. 250C is not too extreme, a bit under 500F, and getting up there towards damn hot but still pretty manageable. For fluorocarbon mp
references PFA 306C FEP 260C and PTFE 327C so PFA or certainly PTFE could be used for plumbing. And hey if inconel or
316 won't do it then grade 1 titanium I have on the shelf too. I'm kidding a little bit here. Actually copper should work fine, and even glass or
ceramic. 250C is not too extreme, a bit under 500F, and getting up there towards damn hot but still pretty manageable. For fluorocarbon mp
references PFA 306C FEP 260C and PTFE 327C so PFA or certainly PTFE could be used for plumbing.
I think copper is also passivated by sulfiding and can be used even for the reaction kettle itself.
Also I am making another reference request for an article that should be illuminating about some technical aspects of the use of petroleum derivatives
for H2S generation. This can be sorted out further, but there is useful information even on the first preview page where they show the optimum
temperature as 250C.
Hydrogen Sulfide Production from Sulfur and Hydrocarbons.
http://pubs.acs.org/doi/abs/10.1021/ie50425a024?journalCode=...
Early references about paraffin say that asbestos was used as a catalyst and some patent references say that limited porosity silica or alumina is
used for some hydrocarbon dehydrogenaton by sulfur reactions, so some chunks of silica cat litter "crystals" could help the reaction, I don't know.
Some of that mineral fiber felt like is used for high temperature insulation for ceramic kilns might substitute for asbestos.
Another reference here reports that the reaction of sulfur with alkanes producing H2S begins in the range of 140-150C
so it would seem the higher temperature of ~250C may not be an absolute figure, and it could vary what is the temperature depending upon the C value
of the alkane.
C14 alkanes bp is 250C and fp is 0C, C15 bp 270C fp 5C
and continuing in the same direction with increasing C value alkanes having higher bp and fp temperatures. My guess would be the lower C value
lighter alkanes would begin reacting with sulfur to for H2S at the lower temperatures, and the more wax like higher C value alkanes are less reactive
and require the higher temperatures to react with sulfur to form H2S.
http://pubs.acs.org/doi/abs/10.1021/ja00277a002?journalCode=...
Thanks to leu for the attached article
Hydrogen Sulfide Production from Sulfur and Hydrocarbons
BTW A.P.I. 20.5 = sp. gr. .930921
Mineral Oil should be an entirely adequate substitute,
vaseline would be next, and paraffin next among the substitutes that may be easier to find than heavy fuel oil.
Attachment: Hydrogen Sulfide Production from Sulfur and Hydrocarbons, R.F.Bacon, E.S.Boe, Ind.Eng.Chem.,1945.pdf (339kB)
This file has been downloaded 1560 times
[Edited on 18-4-2014 by Rosco Bodine]
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Rosco Bodine  |
C14 alkanes bp is 250C and fp is 0C, C15 bp 270C fp 5C
and continuing in the same direction with increasing C value alkanes having higher bp and fp temperatures. My guess would be the lower C value
lighter alkanes would begin reacting with sulfur to for H2S at the lower temperatures, and the more wax like higher C value alkanes are less reactive
and require the higher temperatures to react with sulfur to form H2S.
|
Sounds right, I didn't always pay attention as much as I should have in chemistry class, but what I remember is that in general the more carbon atoms
an alkane has the more stable it is.
Paraffin wax bp >370oC
Vaseline bp ~343oC
Though paraffin wax and Vaseline are less reactive than the lighter (shorter chain) hydrocarbons, their lower volatility is probably an asset.
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Maybe next time you run your H2S generator, try white mineral oil instead of vaseline and see if the H2S does come off at a lower temperature with the
more mobile hydrocarbon having a lower C value alkane series like white mineral oil.
IIRC it is about C15 lower end fraction for white mineral oil, but I'll have to check that. Also if you have a scrap of that mineral wool "kaowool"
or other trade name, maybe pull apart some of that mineral wool fiber to loosen the matting a bit and put a "cotton ball" chunk of that mineral based
fiber in with the mineral oil and sulfur to see if it has any effect as a promoter like a physical catalyst scaffold / wick. If it works to help the
H2S produce more easily at a lower temperature this could be a significant improvement. The lower temperature that the reaction can be run the less
sublimation of sulfur will occur. So the generator may run cleaner. It is a tradeoff at a point because the oil has volatility too, but the oil
would tend to reflux, while the sulfur would accumulate and plug the outlet tube eventually. There are many different specialty grades of mineral oil
and there is probably a particular grade that is "perfect" but which one is best I don't know. But I think the most common grade of white mineral oil
like is available everywhere is probably workable. I found a reference that is putting the alkane value for mineral oils as beginning at C17 for the
lightest, so this should be okay, as that would put the boiling point well above the 250C minimum recommended by that reference attached earlier. It
looks like it should work.
Here is another reference that says carbon is catalytic. Earlier I had mentioned it was reported that rosin and sulfur equal parts heated together
evolve H2S and was mistaken about the turpentine. That mixture is just the two things sulfur and rosin.
Attachment: Production of Hydrogen Sulfide by heating Paraffin and other Hydrocarbons with Sulfur.pdf (356kB)
This file has been downloaded 614 times
Attachment: Hydrogen Sulfide pages chemicalengineer24news.pdf (450kB)
This file has been downloaded 718 times
[Edited on 20-4-2014 by Rosco Bodine]
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
I was at a home improvement center a couple days ago and found some stone wool. It is very popular right now it seems judging by the variety and
quantity available. It is used for insulation and soundproofing and is very fire resistant of course. I didn't want to buy a huge bundle, for $50 or
so, so I pinched a very small amount off the corner of one of the batts from a bundle. I don't really agree with shoplifting, but I don't think the
tiny amount I took will hurt anyone especially since a whole bundle looked to be enough to insulate a small shed.

"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Those last couple of references show that lampblack carbon is catalytic and lowers the reaction temperature and increases the yield, however for the
heavier black oils that already have a free carbon content, lampblack isn't needed
and has no substantial effect. What I think may occur is that if some of the carbonized residue of asphalt is saved from an initial run using just a
white mineral oil and sulfur mixture that is rich in oil so it doesn't end up with a solid chunk of carbon as byproduct, that a portion of the
residual asphalt oil can be saved and mixed in as a catalyst with any fresh batch of mineral oil to blacken it with free carbon containing asphalt as
a catalyst for the subsequent batches. That would avoid having to buy lampblack separately. Also the "black oil" mentioned being a lubricating oil
sounds possibly like what would commonly be 90 weight or better and it may be that stinky diesel like odor gearbox oil, or something heavier.
[Edited on 23-4-2014 by Rosco Bodine]
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
I got the following equation, from the webpage linked to below which has fairly exhaustive information regarding the physical properties of sulfur.
http://www.ogj.com/articles/print/volume-93/issue-42/in-this...
ln(p) = 89.273-13453/T-8.9643lnT
where,
p = vapor pressure of sulfur in pascals
T = temperature in kelvins
According to the above website this equation agrees very well with experimental values for the vapor pressures of sulfur. I have included a jpg of a
graph with the temperatures and corresponding vapor pressures of greatest interest for this process (between ca. 200-300oC). I have also
included a pdf of all three graphs made. There is a broad range graph in kelvins as well as one in degrees Celsius and the narrow range graph in
degrees Celsius previously mentioned.
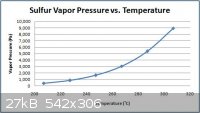
Attachment: Sulfur Vapor Pressure versus Temperature Graphs.pdf (161kB)
This file has been downloaded 3676 times
It is obvious that relatively small reductions in temperature in the hydrogen sulfide generator would drastically reduce the rate of sulfur
sublimation. Regarding the carbon catalyst idea, now that I stop and think about it very early on in the process a lot of carbon is produced as a
product in the reaction between the heavy hydrocarbon and the sulfur.
I think I have found another good use for the stone wool; insulating the delivery tube from the generator to the sulfur trap (deposition vessel). I
found a bit of steel refrigeration tubing which is very thinned walled and much larger in diameter than the brake line. The tube could be insulated or
even heated, for instance by an electric resistance heating element, but really I think it is big enough that it won't plug during the run time I will
be using. A picture of the simple set-up suggested is shown below. The picture does not show any insulation on the large diameter steel tube
connecting the two vessels. Also, the sulfur trap vessel could be placed in an iced bath if it was felt extra cooling was needed to assist sulfur
deposition. The large diameter tube running into the sulfur trap also should have gone nearly to the bottom. The piece of large diameter tubing I have
is not exactly the right length, so it may be replaced in the future.
Small diameter steel tubing (brake line) is ~3.1mm inside diameter
Large diameter steel tubing (refrigeration line) ~ 8mm inside diameter
Therefore, the large diameter steel tube has about 6.7 times the cross sectional area as the small tubing. It will take much longer to plug especially
if insulated and/or heated. It will also be very easy to clean between uses.

[Edited on 26-4-2014 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Your observation about the geometric increase in cross sectional area pertinent to the aspect of "clogging" of a tube by sublimed sulfur is directly
translatable to the clogging and quenching of a detonation wave at near the critical diameter, and shows the thermodynamic effect and energy
multiplication for increased area of a wavefront that can be enormous difference for what is an only slight increase in diameter. Small increase in
diameter can change things drastically at a certain scale, while the geometric difference is not so great as the scale increases well beyond the
dimensions that are near to critical. This is pretty general in all engineering as a transport phenomena or transfer / translational phenomena. Open
up a design constraint bottleneck and there things get much easier fast. Tighten the specs and things get dicey and uncertain as the ceiling for
allowable deviation from optimum is lowered.
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
High Yield Sodium Picramate Synthesis - Experimental
Just to make sure that the results from the last synthesis were reproducible, and to get rid of the last of the sulfide solution before moving, I ran
another experiment. The experiment was performed the same way as last time except that the temperature was a few degrees lower, ~37oC as
opposed to 40oC, at the end of the sulfide-bicarbonate solution addition. After addition was complete supplemental heating had to be added
to bring the temperature up to 40oC or so where the temperature rose quite quickly mostly on its own to ~60oC. This reaction
started much more slowly than it did for the last experiment and overall the reaction time was at least double.
While air drying the sample mass was periodically checked over the course of more than two days and it was found that the mass did not change at all
for the last 12 hours or more at which point the sample was considered to be dry. From 7.52g of picric acid 7.28g of sodium picramate was obtained or
a quantitative yield. The yield is actually about 0.3% over 100%, which just means that there are small amounts of impurities or water still present.
This may not be the best method to measure yield accurately, but I think it is safe to say that the yield is at least very nearly quantitative.

[Edited on 1-5-2014 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Sulfur Trap Model
Here is my idea for a sulfur trap. The trap/vessel could be set in cold or iced water to aid sulfur deposition. The center divider forces the gas to
travel down one side of the trap and up the other before exiting the trap. The center divider could also be hollow and have cooling water flowing
through it. A nichrome resistance wire electrically heated delivery tube could be used to connect the hydrogen sulfide generator to the sulfur trap,
which could eliminate or significantly reduce sulfur buildup between the generator and the trap. I think this arrangement would allow fairly
continuous hydrogen sulfide production. The generator would still need to be recharged with sulfur and hydrocarbon, and cleaned out, from time to time
of course. An alternative to shutting down to recharge and clean could be to simply have more than one generator and swap them out as needed.
I created a simple sketch using Google Sketchup which should illustrate the sulfur trap concept. Attached is a jpg of a top angle view and a bottom
angle view.
 
[Edited on 12-5-2014 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Praxichys
International Hazard
    
Posts: 1063
Registered: 31-7-2013
Location: Detroit, Michigan, USA
Member Is Offline
Mood: Coprecipitated
|
|
Why bother with the hydrocarbon/sulfur method to H2S? You mentioned methods using iron/sulfur earlier, but why not use that just for H2S generation?
Heat iron filings and sulfur in a soup can or something until the reaction starts. Let it cool, then just drip an acid on the FeS with a gas generator
setup. There are no sulfur clogs or messy/stinky organosulfur leftovers to scrape out of glasware, etc.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
There are many valid approaches to generation of H2S and there are many valid alternative reductions of sodium picrate or other picrate to a picramic
acid salt. It would become a matter of convenience what methods would have most appeal based upon what materials are available to then choose how to
proceed with what course for synthesis. There are advantages and disadvantages to various approaches so which methods are best for any particular
scenario would depend on the circumstances of most easily available materials defining the most convenient approach to synthesis for that individual
case.
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Praxichys  | Why bother with the hydrocarbon/sulfur method to H2S? You mentioned methods using iron/sulfur earlier, but why not use that just for H2S generation?
Heat iron filings and sulfur in a soup can or something until the reaction starts. Let it cool, then just drip an acid on the FeS with a gas generator
setup. There are no sulfur clogs or messy/stinky organosulfur leftovers to scrape out of glasware, etc. |
I think I just solved the sulfur clogging issue and really if one is only interested in making enough sulfide solution to reduce ten or twenty grams
of picric acid then simply using a delivery tube that is large enough to not plug during the short run time needed would be adequate. I was using a
brake line for the experiments I posted about in this thread, which was only 3.1mm inside diameter, and while there were some clogging issues I still
managed to produce enough sodium (hydro)sulfide solution for a lot of testing. Obviously glassware which is dedicated for this purpose or disposable
should be used. For instance, I have been using mason jars with a hole cut in the metal lid, using an electricians knockout tool, for the stopper. In
most cases I found that removing the chunk of carbon and organic material from the bottom of the reaction vessel was not that difficult.
The hydrogen sulfide produced by this method is very pure, according to the literature, and it uses very common, cheap materials. The setup I proposed
above would be suitable for producing quite large quantities of hydrogen sulfide, say for example to make a large quantity of sodium (hydro)sulfide
reducer or a large quantity of hydriodic acid.
Like Rosco said, there are many different materials and methods to choose from and their suitability depends on your goals, material availability and
equipment availability, etc.
[Edited on 13-5-2014 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
Hi Henning Brand, congrats with your work on the reduction of picric acid to picramic, some serious progress in this thread!. I must admit I haven't
read every detail of it yet, though I was wondering about one of the earlier articles you posted. Here again they mentioned "sulfur dyes" formed,
especially from aquous solutions and with higher alkalinity. Anyone knowns what exactly they are referring to and to what extent it makes up the final
product after NaS redution? Could't help thinking that it could explain apprent more than 100% yields?
No luck on ascorbic acid/Fe method yet, yields were terrible, almost no precipiation of sodium picramate or picarmic acid after acidification. Seen
the yields the sulfide reduction definitely seems the way to go, though I'm hesitant in directly producing H2S.
Another brainfart that I had was to maybe ignite a 1:1.7 mixture of sulfur and aluminum in a closed crucible to produce Al2S3. Al2S3 in contact with
water produces H2S and Aluminum hydroxide (precipiates). Could you treat the solid Al2S3 with NaOH or NH3 solution and filter to get rid of the
Aluminum hydroxide to produce ammonium of sodium sulphide solution?
[Edited on 22-6-2014 by nitro-genes]
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
Couldt edit my previous post, but some initial optimistic results with aluminumsulfide produciton. 20g Aluminium powder was mixed with 34 grams of
sulfur (slight excess aluminium) and ignited in a stainless steel vessel with small opening to relfief the pressure, that was closed with dry sand to
eliminate oxygen as much as possible (immediately after the reaction). The stainless steel barely survived the reaction, glowing almost white orange
for a full minute after completion. After cooling, the aluminum sulfide was removed from the container with a hammer (this stuff is incredibly
tough!). Then in a narrow glass container, 8 grams NaOH was dissolved in 75 ml of destiled water, after which 10 grams of Al2S3 chunks were added.
Took a couple of hours for the Al2S3 to decompose completely in the NaOH solution, after which a fine layer of presumably Al(OH)3 remains at the
bottom. The decomposition of Al2S3 in water is clearly audible as pronounced H2S bubling can be heard as long as the reaction is not complete. After
filtering a clear, slighty yellow solution of NaHS was obtained. Quick test indicate direct formation of red colour after introducing to small amount
of PA. When kept absolutely dry, the Al2S3 could be stored as a on demand reducing agent.
Cannot believe how easy this seems to work after obtaining Al2S3, I was afraid that the Aluminium hydroxide would form a sort of gel or otherwise
difficult to seperate fine precipitate, but it can actually be filtered out really easy.
Will try larger scale reduction tomorow. 
Edit: The reaction vessel used for the Al+S reaction containing remaingin Al2S3 is best filled with strong NaOH solution to eliminate H2S formation,
don't use water!
[Edited on 24-6-2014 by nitro-genes]
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Thanks for the high praise. I am more of an explosives and science/technology enthusiast than a chemist, but I will point out a couple things that
seem relevant.
This first attachment called "Communications" provided by Rosco, talks about yield and how the reduction should be carried out between 50 and 60
Celsius for best yields of the purest product. High temperatures (e.g. 70oC) as well as the presence of excess hydroxide ion in the
reaction mixture are also reported to reduce yield. Possible undesirable by-products are also described. There are at least two or three sources
posted in this thread which describe by-product dye formation during the reduction, and they all describe them differently. What they all seem to
describe, though, is a lessening of product weight recovered from the reaction mixture and a darkening of the solution as the quantity of by-product
formation increases.
See article titled “Communications” posted as attachment.
http://www.sciencemadness.org/talk/viewthread.php?tid=433&am...
See Hodgson and Ward article posted as attachment.
http://www.sciencemadness.org/talk/viewthread.php?tid=433&am...
I am sure that there are other ways to produce an effective reducer for picric acid. Except for the serious poison danger, generating hydrogen sulfide
by heating a heavy hydrocarbon and sulfur is very advantageous. It allows very pure hydrogen sulfide to be produced and as long as high purity sodium
hydroxide is used the sulfide reducing agent produced will also be very pure. The composition of the reducing solution produced can be easily
determined by taking the weight of the sodium hydroxide solution before H2S absorption and after. The old expression, “garbage in,
garbage out” I think applies here. Most of the reducing agents, I have seen used, seem to be a mixture of various reducing agents and unreacted
material, etc. I do understand the desire to avoid the use of hydrogen sulfide gas though. It is quite unpleasant material and it can be downright
dangerous if not handled properly. Your method of reacting aluminum and sulfur seems good. Interesting to see what kind of results you get when
reducing picric acid.
[Edited on 24-6-2014 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
Today I tried the reduction of picric acid to picramic on a larger scale, it seems Al2S3 is realy conveniant for the production of NaHS solutions.
Al2S3 was produced from direct reaction of Al with S as desribed earlier. It appears as black chunks in the picture, probably due to contaminants from
the dark german aluminium used. (aluminium carbide, carbon etc)
8 grams of NaOH was dissolved in 75 ml of destilled water. To this was added 10 grams of Al2S3 chunks. The Al2S3 immediately starts to decompose as
conitnued bubling can be observed opon adding the NaOH solution (photo). It takes several hours to decompose the Al2S3 completely and was kept in the
fridge overnight. After complete reaction, the resulting NaSH solution smelled slighlty of H2S, indicating that mostly NaHS and a small amount of free
H2S were in solution.
After overnight in the fridge, the aluminium hydroxide/oxide and contimants were mostly at the bottom of the beaker (photo) and could be filtered of
easily. A small amount of wash water was used to retrieve all of the NaHS. After filtering off the precipitate, a slighly yelowish solution is
obtained (photo).
Next, 15g of recrystallized picric acid was dissolved in 100 ml's of NaOH solution (2.62g NaOH). This was heated to 50 deg C to dissolve all of the SP
and prevent precipitation from solution upon addition of the NaHS solution. Slow additions of the NaHS solutions were made, so that the temperature of
the solution remained between 50 and 55 deg C. A slight amount of what appears to be sulfur was forming on the boundary of the solution and air,
visible as a slight opaque film on top of the solution.
After 3-4 minutes most of the sodium picramate precipitated. The solution was kept in the fridge untill at 5 deg C. The resulting sodium picramete was
washed twice with an icecold 10% salt solution, and finally icecold water. Yield looks pretty good, though it needs to dry first. Upon heating it
puffs off pretty violent, only the slightest smell of buring sulfur can be noticed.
       
[Edited on 24-6-2014 by nitro-genes]
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
Regarding the dark brow solutions upon alkalinity increase... Noticed similar dark brown colour of the solution after attemped 8 hour ascorbic acid/Fe
redution and hardly any precipiation of picramate/picramic acid. I'm beginning to suspect that what they call sulfur dyes may actually be nothing more
than over reduction of picramate into di ,-and triaminophenols. Ascorbic acid is certainly capable under certain conditions to produce the diamino
variant, it has even been patented if I'm not mistaken.
[Edited on 24-6-2014 by nitro-genes]
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
oh, almost forgot... it also makes some realy fast DDNP 
Attachment: P1030094.avi (4.1MB)
This file has been downloaded 821 times
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Nice pictures, looks like you have had success. That's a pretty clever idea using Al2S3. Interested in hearing what kind of yield you got.
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
Won't be able to weigh the sodium picramate for yield... I didn't want to store the sodium picramate itself since it deflagerates pretty violent and
for the DDNP synthesis picramic acid was needed. So I went ahead and dissolved al of the wet sodium picramate in the least amount of 80 deg C water.
Used the suggestion of Rosco, by slowly (while stirring) adding 10% HCl solution to precipitate the picramic acid over the course of about 15 minutes.
The picramic acid precipitated this way does not form the usual mud like substance but filters more easily. Still pretty fine crystals but a closeup
look seems to suggest ~0.2 mm long needle like crystals that glitter in reflective light. There is a pretty sharp point at which most of the picarmic
acid will come out of solution, could have been more patient in the HCl addition to maybe produce even larger crystals. To my surprise, the picramic
acid obtained definitely looks more red than brown as I obtained usually, don't know if this is a purity issue or relates to crystal size as well.
The picramic acid will be weighed tomorow (or the day after), when it is dry.
[Edited on 24-6-2014 by nitro-genes]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
About this I am not certain, but possibly it is worth experiments to see if picramic acid is useful as a gasoline additive for dissolving and reducing
levels of rust trapped in fuel filters and fuel tanks corroded by ethanol or methanol or other oxygen containing fuel additives and moisture
contaminated fuel. A related patent GB1574297 is attached.
And the U.S. issue patent US4145190 is also attached.
Also attached is US4073626 which specifically mentions picramic acid, column 3 line 18.
Attachment: GB1574297 Ferrous Picrate Fuel Additive.pdf (516kB)
This file has been downloaded 611 times
Attachment: US4145190 Ferrous Picrate Fuel Additive.pdf (423kB)
This file has been downloaded 582 times
Attachment: US4073626 Ferrous Picrate Fuel Additive.pdf (331kB)
This file has been downloaded 593 times
[Edited on 25-6-2014 by Rosco Bodine]
|
|
|
| Pages:
1
..
7
8
9
10 |