| Pages:
1
..
8
9
10
11
12
..
33 |
Ral123
National Hazard
   
Posts: 735
Registered: 31-12-2011
Member Is Offline
Mood: No Mood
|
|
Very interesting, in my experience HMTD is the only thing that explodes if you walk over it. For the DDNP I suspect it's physical from can be the
difference between very useful and barely suitable for basecharge.
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
I normally try not to leave primary explosives around where they will be walked on. Actually, I am extremely careful to not leave primary explosive
around where they could be the cause of an accident. The people who did the testing for the report, just referenced, used a BAM testing apparatus
which is a fairly precise method of friction testing, from the little bit I have read about it.
What you are saying about physical form is not correct in my opinion. There are references posted earlier in this thread with information related to
this. I would not go as far as to say that physical form has nothing to do with performance, just that it is not the most important variable by any
means. I believe that improved handling properties are probably the biggest reason for investing energy into modifying particle size and shape.
There is a lot of talk in the recent literature about spherical DDNP powders of fairly large particle size. I have been looking into it a bit, but the
processes usually involve an added crystallization modifier, usually a type of dye. I don't believe that these special spherical powders are necessary
in terms of performance, by any means, but they most likely have excellent handling properties and would make automated loading much easier.
On a side note, I have started wearing a dust mask when working with picric acid, DDNP and other toxic powders that can and do become air born at
least to a small degree during handling.
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Ral123
National Hazard
   
Posts: 735
Registered: 31-12-2011
Member Is Offline
Mood: No Mood
|
|
The major problem of DDNP is it's DDT length, so I guessed the particle shape, size, density and loading pressure can have a large effect. Who has
done HMTD burn test on the ground and small parts were left, knows how easily it pops when you walk over it.
Speaking of stability, can anybody suggest if these are in the right order of storage stability
TNB>TNP>RDX>Tetryl>DDNP>MEKP>ETN>MF>HMTD?
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
I think we may be talking past each other. I assumed by physical form you were talking about specific surface (i.e. particle size). Something that I
don't think I acknowledged earlier on in this thread, when it was discussed, was that having the right particle size can make the loading tolerances
much better (will tend to perform better even if not at correct loading density, in particular when below the optimal density). However, as stated
earlier, with DDNP if the powder is loaded to the correct density it makes little difference whether it is fine powder or coarse crystals. Also with a
very small unequivocal primary such as lead azide pressed on top of the DDNP, the DDNP can be pressed very hard and it then becomes a fantastic
initiator. In my last test I used only 0.30g of DDNP and 0.05g of lead azide to initiate picric acid in 7.6mm thin walled aluminum tubing, with no
reinforcing cap, and the output was extremely high indicating that the primary explosive weights could very likely be dropped even more and still
achieve very good results.
Loading pressure and density are directly related.
The strengths and limitations of DDNP I think have been fairly well examined and defined in the last few months in this thread. Read through some of
the test results that I posted; I think I have done about 14 tests now.
Why don't you do the tests and try and prove your theories? Until you do some testing, or find a good reference, they are not worth that much.
I have made HMTD quite a few times and when I lit it, it was all gone in an extremely fast flash. You must have had it spread out
pretty well, either that or it wasn't HMTD. Well, the more I think about it, I do remember occasionally seeing little bits of HMTD left after a test,
but I always ignited them for fear that they could be a hazard to others or to me when I was doing another test in the future, possibly with a
different explosive, in the same location.
BTW, I have been working on a recrystallization method today for DDNP. I will post pictures soon.
I have to ask, how many different primary explosives have you walked on? Was it always on the same surface and were you wearing the same shoes, etc,
etc?
[Edited on 20-5-2014 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Ral123
National Hazard
   
Posts: 735
Registered: 31-12-2011
Member Is Offline
Mood: No Mood
|
|
If we'll be adding small amounts of azide on top of DDNP, I can add small amounts on top of RDX or MHN, it will be much more effective, I guarantee
it.
The mine field effect doesn't happen with azide and AP.
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
DDNP Recrystallization by Capillary Action Addition of Water to Acetone Solution
The idea of using a wick is not a new one. The idea of transferring a fluid in a controlled manner from one vessel to another using a wick has been
mentioned here and at the old E&W forum. I decided to give it a try since unlike evaporative recrystallization, it would purify the DDNP and would
not put a lot of acetone vapors into the air. I first tried a piece of loosely rolled up paper towel, to transfer water from one bowl to another. It
took about one hour and fifteen minutes to transfer about 50mL of water, in such an even and slow manner that no human could match it with a pipette
and if they tried they would be driven to frustration and then madness. 
  
I next set up a homemade stirrer and a beaker with 2g of DDNP dissolved in ~40mL of acetone, a plastic bowl with water and the same wick from earlier
connecting the two. A tiny amount of DDNP fell in the water, which is why it is colored. This time it took about 3 hours to transfer 50mL of water,
since the wick was held more vertically, placing more gravitational force on the water in the opposite direction from water travel up the wick.
  
It worked quite well but the sample is still a bit damp. I want to try and get microscope pictures of the unrecrystallized and recrystallized material
by tomorrow for comparison purposes.
 
It is obvious that there was a significant amount of weight loss again. If it really is mostly impurities that are being lost, is it really a big
advantage to lose them? Most of the impurities present are probably quite energetic as well. I suppose the purified material would probably have much
better storage stability.
[Edited on 20-5-2014 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Ral123  | If we'll be adding small amounts of azide on top of DDNP, I can add small amounts on top of RDX or MHN, it will be much more effective, I guarantee
it.
The mine field effect doesn't happen with azide and AP. |
Those are secondaries, and DDNP is a primary albeit a bit slow to start one. All it takes is a tiny snap from lead azide to get DDNP into high gear
though. The other option is also the reinforcing cap with DDNP. DDNP either requires strong confinement or a little help from an unequivocal primary
explosive like lead azide. Silver azide would be even better probably, because it makes DDT in even smaller quantities than lead azide. By using
silver azide it would probably be possible to get away with only 0.01 or 0.02g on top of the DDNP. It will take much less azide to properly initiate
DDNP than any of the secondaries. DDNP is made from picric acid and other useful materials can also be made from picric acid. All of these picric acid
related syntheses require only commonly available reactants for the most part. Picric acid is much more storage stable than even RDX which is by far
the most stable of those you listed. I could write several pages on the advantages of picric acid, if I felt like it. Here is one more big one, azide
economy. If I use 0.02g of silver azide per detonator, I can make 50 detonators from 1g of silver azide. These detonators will be quite safe as well
in my opinion with only 0.02g of sensitive silver azide in them. Picric acid is very insensitive and DDNP is one of the most insensitive primary
explosives (except to heat or flame). Picric acid doesn't have the power of RDX or the solid nitric esters, but its brisance is still very high and it
makes a fairly good initiator and it also is incredibly versatile.
The big thing is that I like a challenge and I also feel that these materials are useful and deserve a place in my "toolbox". I have access to PETN,
and it is arguably the best base charge material in most ways, but picric acid and its derivatives have their place too and they collectively probably
take about 100 times more time and effort to master than PETN for instance.
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
140 pounds of pin pressure should be about optimum for 14 MPa or 2000 p.s.i.
that should be about the "sweet spot" for loading compression of DDNP at 7.6 mm column diameter.
|
|
|
Ral123
National Hazard
   
Posts: 735
Registered: 31-12-2011
Member Is Offline
Mood: No Mood
|
|
The methods of recrystalization I know are adding acetone solution to weak bicarb solution or letting acetone solution evaporate partially. I guess
the first neutralizes, the second purifies. The first procedure alone made very stable Tetryl. I guess you're looking for very pure product, no matter
the loses.
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Rosco Bodine  | 140 pounds of pin pressure should be about optimum for 14 MPa or 2000 p.s.i.
that should be about the "sweet spot" for loading compression of DDNP at 7.6 mm column diameter. |
Yeah that's about right I think. I just calculated 142.4lbs for a 7.6mm pin and it made me question what I did before. Before I had calculated 100lbs
of force, but checking back I see that I was calculating for a 1/4" loading dowel, which would need about 99.4lbs of force to give the 14 MPa of
pressure (sweet spot pressure). I suppose I was off a bit because it would depend on the diameter, or cross sectional area, of the column of
explosives as well since the explosive will tend to hang together even if the loading dowel or pin is a little undersized.
Yield from Capillary Action Recrystallization Experiment
Just took the yield from yesterdays capillary action solvent addition recrystallization experiment. From 2g of crude product, which was made by method
B from the article, "DDNP a Detonating Explosive", 1.3g of very pure crystalline product was obtained or about a 65% yield based on the crude DDNP
started with. The filtrate is an extremely dark brown with a bit of red to it (I am not great at describing colors).
Below on the left is a 100X microscope picture of the unrecrystallized product, which no matter how hard I tried to focus in on it or prepare the
sample I could not make look like anything other than little clumps. BTW, this crude material is what I have been using for the last few explosive
tests. The middle picture is a 100X microscope shot of the recrystallized material. It has very well formed large crystals of yellow DDNP. I may have
to try 0.2g of this material initiated with 0.05g or less of lead azide or silver azide to initiate 2g of picric acid. The picture on the right is of
the filtrate.
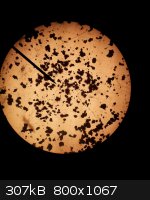  
BTW, the DDNP produced using method B from, "DDNP a Detonating Explosive", is not really in as nice a form as I indicated earlier in this thread.
http://www.sciencemadness.org/talk/viewthread.php?tid=439&am...
It is more or less free flowing, but has fairly low density and is quite fluffy and dusty. Although I would have to go back and check that I followed
their procedure exactly to the letter.
[Edited on 20-5-2014 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
There may be a better scheme for recrystallization not using water at all, but using mixed solvents. Try disrupting the acetone solution with naptha
or xylene or toluene. Another solvent I have wondered about is nitromethane in which picric acid is very highly soluble even to forming a 70% PA
solution, and I wonder what may be the solubility of DDNP in nitromethane. It could react though, as DDNP does react with some solvents like
isopropyl alcohol. One of the patents described precipitating DDNP dissolved in acetone by addition of gasoline. I was thinking the solubility of
sulfur in xylene would tend to eliminate any sulfur impurity by holding it in solution if the DDNP had low solubility in xylene.
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Could be some good ideas there. Another thing is that in order to run the above experiment at room temperature quite a large amount of acetone was
needed compared to what would be needed if the acetone was near the boiling point (I don't have that exact solubility data though). This would
contribute to a reduced yield. Maybe it should be a two step process. Use a much smaller amount of hot acetone, as opposed to room temperature
acetone, and then drown in ice water to precipitate the purified DDNP (as described in "Military Explosives"). If larger better formed crystals were
desired, evaporative recrystallization could be performed or some other recrystallization method that I have not seen yet.
Right now I am letting the acetone evaporate from the filtrate in a dark location. Maybe a lot of the impurities can still be held in solution if only
a certain portion of the acetone is removed by evaporation.
I tried adding gasoline to the filtrate and I ended up with two layers of course, but also an emulsion. It could have been my fault because I agitated
the mixture a bit after adding the gasoline.
[Edited on 20-5-2014 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Thermal Stability of DDNP
Here is a journal article which examines the thermal stability of DDNP. They extrapolated from experimental results to obtain results for the extended
time stability data, but their methods seem very good. The estimated time that DDNP would store for with only 0.6% mass loss was found to be at least
40 years at 50oC. However, if the temperature was increased to 60oC, 0.6% mass loss happens in 5.5 years and at
100oC, 0.6% mass loss occurs in just 2 days. It was stated that 0.6% mass loss can be accepted for all uses of DDNP. It is obvious that at
ordinary storage temperatures DDNP is in fact very thermally stable, but as temperatures increase above 50oC thermal stability quickly
declines.
Attachment: Thermal Stability of DDNP.pdf (487kB)
This file has been downloaded 1097 times
[Edited on 21-5-2014 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
A couple of pages before in this thread it was in the patent US1460708
http://www.sciencemadness.org/talk/viewthread.php?tid=439&am...
where it was mentioned a warm acetone solution of DDNP could be added to gasoline. Coleman fuel is a white gasoline with no additives so it would
likely work as the contemporary "gasoline" equivalent of that circa 1919 patent.
To me however it would seem the order of addition would be reversed, adding the gasoline to the warm acetone solution little by little.
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Static Sensitivity of DDNP
Found a little information on DDNP static sensitivity, though in the body of the report the authors state that the sample was old and they didn't
verify its purity. The report is titled, "The Electrostatic Spark Sensitivity of Bulk Explosives and Metal-Oxidant Mixtures", and can be found in the
DTIC database. I have made jpgs of the introduction and the table which shows DDNP's static sensitivity curves in relation to several others. The copy
is an extremely poor one, but it is still readable for the most part.
 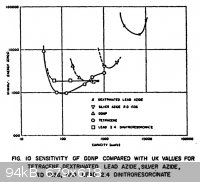
On the x-axis of the graph is capacity in micro-microfarads (picofarads), and on the y-axis is static energy in ERGS.
[Edited on 21-5-2014 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
2.0g of Picric Acid Initiated with 0.20g of DDNP & 0.05g of Lead Azide (Unreinforced Configuration & 6000psi Loading Pressure)
The loading force was measured this time and found to be about 420lbs, and it would be similar for the last couple of tests as well since it was the
same lever, the same loading dowel and the same person was using them in more or less the same way. The loading dowel I have been using for all the
tests except for the possible exclusion of the first one or two is a 5/16" hardwood dowel that was sanded down so that it can slide freely in and out
of the 7.6mm aluminum casings. The loading force of 420lbs corresponds to about 6000psi (~41 MPa) of pressure on the loading dowel. The picric acid,
DDNP and lead azide were all pressed using approximately this same pressure. The DDNP used was the recrystallized material from the capillary action
recrystallization experiment.
There was a complete detonation, but a clean hole was not blown through. The piece of steel left in the hole is very thin, however, and is cracked
through in several places. The steel scab came off the bottom of the witness plate at very high velocity and blew a hole in the ground over 2 inches
deep.
The holes on the right, in the witness plate shots, are from this test.
   
 
During the test I noticed the end of a casing still packed full of picric acid from the last failed detonation. The piece of cap can also be seen in
one of the pictures.
[Edited on 22-5-2014 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
I think you are excessive with the loading pressure and are losing performance for that excess. I think you already posted this chart earlier so you
probably know this data.
Have you tried a loading pressure at 140 lbs pin force?
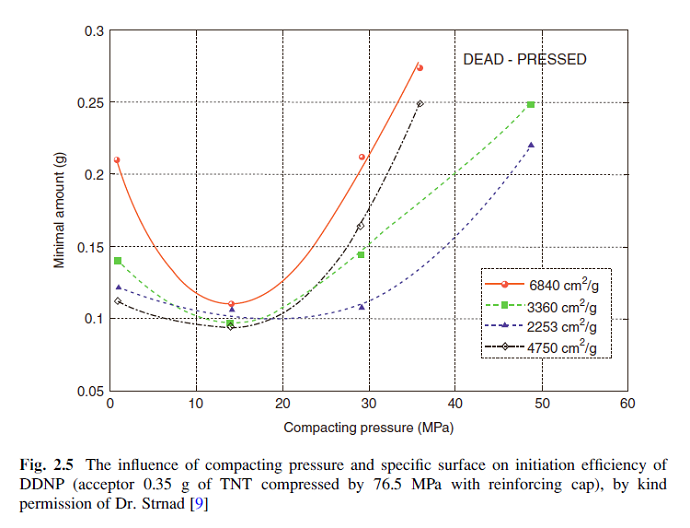
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
I haven't forgotten about that, but I have a theory that DDNP initiated by fuse and DDNP initiated by a supersonic shock wave from lead azide, or
another unequivocal primary explosive, are two very different scenarios. I could be wrong, but it would seem that reducing density, increasing
confinement, etc, would be of much greater importance when DDNP was simply lit by black powder fuse or electrical igniter than if shocked by lead
azide or a lead azide type explosive. By initiating DDNP with lead azide, I think it eliminates most of the precise loading requirements that are
needed in order to get DDNP to self accelerate as rapidly as possible when simply lit. The fact that the reinforcing cap has been abandoned since
adding small quantities of lead azide, and the minimum primary weight requirement is still about the same, is very telling I think. I would like to
hear your thoughts on this.
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
To an extent that is true, however the chart is for reenforced cap equipped detonators, which tends to be a parallel effect. There is some self
compression by the DDT under a reenforcing cap that will shift to the right some the values for what may be optimum but I doubt it would triple the
values. It might bump from 14 to the 17 to 20 range, maybe 170 to 200 pounds pin force. Whatever you add in compression is going to make the base
charge harder to initiate so you have something of a tradeoff, that tends to offset the gain you get and makes a parallel effect of increasing the
minimum initiating charge as well as increasing the amount to overdrive the initiation which is what you are trying to do. Increasing the diameter to
5/16" which is where you are or actually a bit under at .3" allows for lowering the loading pressure and at 3/8" it would actually be into hand
pressing range. For picric acid or styphnic acid base charges at 1/4" I was using 8,000 p.s.i. on the base and 6,800 p.s.i. on colloidal pure Pb
Azide as an incrementally increased kind of sweet spot by testing at that diameter in a very strong laminated aluminum lined brass casing 5/16" O.D.
which is more highly tuned than a brittle Al alloy arrow shaft casing at about that 5/16" I.D. I know from increasing the I.D. and lowering the
strength of the capsule that the device is detuned WRT to loading pressure requirements while the economy of use of the minimum required amount of
initiator is a cost paid for the change, but which lowers substantially the tuning requirements for materials and pressures. The above tests were
without any reenforcing cap. Generally the loading pressure is more critical in tuning for the smaller particle size intitiator to be optimized and
there is a correlation for that pressure and particle size that is parallel whether it is a DDT scheme initiator or an unequivocal initiator, which is
odd but holds true for DDNP and Pb Azide, and I think it probably holds true also for base charges. When the pressure is raised beyond a point it does
increase the brisance but the penalty in requiring larger minimum initiator also to realize the higher velocity is a tradeoff that works counter to
the economy when there is work done to try to lower the minimum required amount of initiating charge. So it isn't worth the added 10% output from the
base density increase if you have to increase by a third the minimum initiating charge to get the more highly tuned configuration. At that point it
is easier just to increase the amount of base charge for more output at the lower compression but easier to initiate base charge. These parameters are
particularly interactive in a detonator scale device, but at a much larger scale these effects will not be seen as so tightly calibrated and will
become more generalized effects. There is another chart in other literature which I can't specifically recall that was showing the correlation holds
whether a reenforcing cap is used or not even though the figures are different being lowered for the minimum initiator charges for the reenforced
configuration. The effect I think is likely attributable to compression heating that attends DDT processes and also that heating makes the base
charge more sensitive to initiation, so there is a gain gotten there by leaving the firing train some space to do its own work of self-compression
almost like a diesel effect causing a multipoint detonation from a thousand little origination points throughout the charge. Hit something
compressible and heat sensitive hard with a hammer and it gets hot enough in a microsecond to cook off. A detonator is like a pile driver in a can.
[Edited on 22-5-2014 by Rosco Bodine]
|
|
|
Ral123
National Hazard
   
Posts: 735
Registered: 31-12-2011
Member Is Offline
Mood: No Mood
|
|
Is there a way to dead press azide-DDNP-RDX at 7mm diameters? It's hard to imagine, but if it can happen, how about MHN instead of RDX? Cast ETN will
fail or be weak and unstable.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
"dead pressing" is kind of a misleading term that describes a counterproductive performance bump on a graph showing a loss of initiating efficiency
under the condition. It is still very definitely detonable and at increased velocity but is a hell of lot harder to get to pop. It doesn't mean at
all that the material is not still very much explosive, just way much harder to get going. Think of dead pressing as an identified "region of
difficulty" where you need a bigger hammer.
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Ok, I understand what you are saying about compressive heating, etc, for the less dense material. I should probably do another test with the same
materials and quantities at the lower density and see what happens. We are getting into some fairly fine points at this stage though I think. I am
still interested in increasing my understanding and seeing how far we can go with this material, but at the same time I am very satisfied with the
results so far. DDNP has proven itself to be a very useful explosive material in my opinion.
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
A good example of the tradeoff is comparing cap sensitive emulsion explosives, where the exact same formula composition is not cap sensitive at a
higher density but requires sometimes even a substantial booster and attains a higher velocity, but when the emulsion is aerated, being foamed to a
lower density it becomes cap sensitive and yet its ultimate velocity is lowered. The same effect occurs with detonators.
|
|
|
Hennig Brand
International Hazard
    
Posts: 1284
Registered: 7-6-2009
Member Is Offline
Mood: No Mood
|
|
Adiabatic Compression Ignition (a.k.a. The Diesel Effect)
The Fire Piston
This is maybe a little off topic, but I think these little devices do a wonderful job of demonstrating compressive heating. I have built several of
these using various materials, and the one shown in the picture is the last one. I was at a yard sale a few years ago and I noticed a box of stainless
steel hardware with $10 marked on it. Being interested I dug through the box and at the bottom of the box, and accounting for more than half the
weight of the contents, were long 1/4" SS bolts with only a small amount of thread on one end and close fitting SS sleeves to go with them. On
inspection the sleeves were found to be seamless, so of course I bought the box. IIRC there were about 30 of these bolt sleeve pairs in the box.
I just dug out my fire piston and took a few pictures. There are lots of YouTube videos of people demonstrating fire piston operation. The tinder I am
using is a type of tinder fungus called the Chaga mushroom. About eight or ten years ago, after learning about the Chaga mushroom, I would always stop
to collect it whenever I saw it in the woods. My parents started to complain after I had three large cardboard boxes full of it in the living room.
Whatever happens I will always have lots of tinder fungus. 
Adiabatic compression is compression were theoretically no heat is transferred to the surroundings. This is a very close approximation of what happens
when there is rapid compression like in a diesel engine, a fire piston or especially in a blasting cap. The effect is so rapid, that a negligible
amount of heat escapes. I think a fire piston makes a wonderful demonstration of this concept. Simply using human power to compress a gas (air) to
above the ignition point of tinder makes a very interesting demonstration. IIRC temperatures often reach several hundred degrees Celsius in a fire
piston, for a brief moment, at the point of maximum compression.
 
  
[Edited on 23-5-2014 by Hennig Brand]
"A risk-free world is a very dull world, one from which we are apt to learn little of consequence." -Geerat Vermeij
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
And the cave man said, Smack! Let there be fire! Fire in the hole! It's magic! Now we're cooking.
If a little nitroglycerin was soaked into that ball of tinder ......kaboom.
[Edited on 22-5-2014 by Rosco Bodine]
|
|
|
| Pages:
1
..
8
9
10
11
12
..
33 |