| Pages:
1
..
25
26
27
28
29
..
33 |
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Quote: Originally posted by PHILOU Zrealone  | Quote: Originally posted by Rosco Bodine  | Rosco also believes that for DDNR the diazo-oxide forms straight across the ring as a para structure, not bonding with the hydroxyl that is ortho,
adjacent as occurs for o-DDNP, but like the structure of the diazo-oxide found with p-DDNP. The tension across the ring bends the entire ring
structure like a hexagonal spring wave washer being bowed by tension across its opposite vertices at para positions 1 and 4, and the diazo-oxide
structure is the bowstring.
[Edited on 2/21/2017 by Rosco Bodine] |
Simply ask and voilà added 
The structure of trans-diazo is stil a bit mysterious...in reallity it must display a trans quinonoid structure so that both sides can bend onto the
same side of the paper plane while the diazo connects to the opposite side...but connectivity is a bit hard to figure out. Into the book Traité de
chimie organique Azoiques-diazoiques,.... they mention that the probable connectivity passes via a direct link between the N2 and the Cs on both sides
thus without the oxygen being involved...a bit a cage structure related to DABCO(DiAzaBiCycloOctane) but with the Ns at other places and with
unsaturations...anyway this is highly stressed...
|
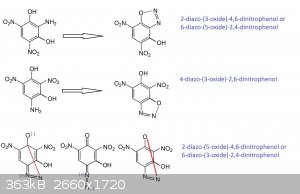
That looks good except for the first entry should be 2-diazo-(1-oxide)-4,6-dinitrophenol instead of the 3-oxide you show.
The middle compound is synonymous with what Klapotke called HODDNP
Looking at the last listing, the 6-diazo-(3-oxide)-2,4-dinitrophenol nomenclature would work there for the structure inverted and rotated
counter-clockwise 30 degrees.
It is the stressed structure that accounts for the surprising energy as an initiator compared with an ordinary styphnate.
Anyway, the convoluted nomenclature is in part or completely explained by not associating the compound with resorcinol as a derivative.
I think that different researchers working from different precursors and not making the association with resorcinol is why the failure to make
attribution to the earlier references about DDNR that trace back to Benedikt and Hubl in 1881.
It could be the NMR data is correct but is referenced to structure nomenclature for phenol rather than resorcinol, then having all the expected
resorcinol associated position numbers made into alternate expressions. Also, Klapotke identifies ortho bonding with the adjacent hydroxyl to the
diazo as occurs with the usual o-DDNP. But the scenario we have involving p-DDNP is more similar and strongly suggests a diazo-oxide that is the same
as DDNR, even though it would seem either structure is possible.
[Edited on 2/21/2017 by Rosco Bodine]
|
|
|
Marvin
National Hazard
   
Posts: 995
Registered: 13-10-2002
Member Is Offline
Mood: No Mood
|
|
PHILOU, Where did 2-diazo-(3-oxide)-4,5 dinitrophenol / 6-diazo-(5-oxide)-2,6-dinitrophenol come from? I didn't write that. Who thinks that is the
structure?
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Thanks to Louis for the synonymous expressions. In the context of the alternative synonymous naming for DDNR, the issue with the NMR data for the
diazo position is reconciled as not being a problem about the NMR data but the differing nomenclature, recognizing the same NMR data could correspond
with a diazo at position 6 or 4 described by different diagrams orientation corresponding to different long names and "parent" compounds. There is
still a question about the diazo-oxide configuration, whether it is the hydroxyl oriented ortho or para to the diazo, that becomes an anhydride in
forming the diazo-oxide.
The differing nomenclature for the same DDNR compound seems to be the best explanation why all the older prior art references were not included by
Klapotke, and why the DDNR is being called a "hydroxyphenol" which is technically correct, but diverts attention away from the prior art for DDNR
referenced as a derivative of resorcinol.
Making this still more confounding is that isomers are possible, for the DDNR, although in this particular case I believe the compound [8] identified
by Klapotke is the same compound identified in 1881 by Benedikt and Hubl, later by Mendola and others.
I think the uncertainty of which hydroxyl (ortho or para to the diazo) is intact and which hydroxyl becomes the anhydride associated with the diazo
has contributed to the confusion.
I think these 2 linked posts were the earliest posts where I was questioning the structure being identified, and saying I thought the hydroxyl and
anhydride were transposed
http://www.sciencemadness.org/talk/viewthread.php?tid=439&am...
http://www.sciencemadness.org/talk/viewthread.php?tid=439&am...
The significance of what I am trying to say is that Klapotke's compound [8] and DDNR are one and the same.
Based on experiments reported by nitro-genes there is a niche reaction condition that allows for (probable) DDNR to be formed from a paracetamol
precursor, without having to first further acetylate paracetamol to a diacetyl derivative.
The interesting and bizarre story here is that over a period of 134 years, four or five different researchers have made the exact same compound,
(DDNR) by different methods and called it by different names, "reinventing the wheel" as if it were invented for the very first time  And it seems nitro-genes has added another chapter to the story, not yet fully
sorted out. And it seems nitro-genes has added another chapter to the story, not yet fully
sorted out.
[Edited on 2/21/2017 by Rosco Bodine]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
While the niche reaction conditions for best yield of DDNR from paracetamol not further acetylated before nitration are an interesting unexpected
development reported by nitro-genes, how efficient will be that approach is unknown and may not ever work as well as proceeding from paracetamol
acetate, which is Klapotke's compound [5].
It appears likely to me that the N NMR data identifying a diazo at position 6 for compound [8] of the Klapotke article is explained by nomenclature
semantics that have inverted and rotated positions so that Klapotke's position 6 diazo for Klapotke's compound [8] (and likewise for unrecognized [8]
is DDNR) is synonymous with what I am calling position 4 for the same diazo and is the same compound, DDNR, with confusion over nomenclature arising
at compound [5] in the Klapotke article where p-aminophenol is converted to a diacetyl derivative that is O,N-diacetyl-p-aminophenol, or paracetamol
acetate, or 4-Acetamidophenyl Acetate, but is identified synonymously by Klapotke as N-(4-Acetoxyphenyl)acetamide, which inverts the subsequent ring
position numbering and causes the nomenclature confusion thereafter. Other confusion of the structure numbering would have occurred for nomenclature
like 4'-Hydroxyacetanilide Acetate.
For example, in the alternative, if Klapotke's compound [5] had been named O,N-diacetyl-p-aminophenol and obtained as at least 100 grams yield from
97 grams of paracetamol substituted for the 35 grams of p-aminophenol described which yielded only 50 grams instead, everything to follow would have
been simplified, efficiency would have improved and the nomenclature confusion about the 4 versus 6 diazo would have been entirely avoided.   
Likewise, it is the potassium derivative of Klapotke's compound [8] that is of particular interest for testing initiator properties identified by Von
Herz and physical properties identified by Benedikt and Hubl, Meldola, et al, in order to ascertain if indeed the compound [8] of Klapotke is DDNR as
I believe it is.
Klapotke's compound [8] 6-diazo-3-hydroxy-2,4-dinitrophenol (HODDNP) is I believe the same DDNR identified by Benedikt and Hubl and Meldola and
others.
Here attached again are the two Klapotke articles
Attachment: Synthesis & Energetic Properties of 4-Diazo-2,6-dinitrophenol and 6-Diazo-3-hydroxy-2,4-dinitrophenol.pdf (286kB)
This file has been downloaded 700 times
Attachment: Synthesis and Initiation Capabilities of Energetic Diazodinitrophenols.pdf (754kB)
This file has been downloaded 899 times
[Edited on 2/22/2017 by Rosco Bodine]
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by Marvin  | | PHILOU, Where did 2-diazo-(3-oxide)-4,5 dinitrophenol / 6-diazo-(5-oxide)-2,6-dinitrophenol come from? I didn't write that. Who thinks that is the
structure? |
You indeed wrote:
6-diazo-3-hydroxy-2,4-dinitrophenol aka 6-diazo-2,4-dinitroresorcinol
and
4-diazo-2,6-dinitroresorcinol.(*)
Those are ambiguous names as seen in the practice by all of us when trying to understand each others between forum members, but also when trying to
understand and correlate the research of Klapote's team and previous early works from the past.(**)
So your wrongly named and ambiguous 6-diazo-3-hydroxy-2,4-dinitrophenol is the same as 6-diazo-3-hydroxy-2,4-dinitrophenoxide (an ortho diazo-oxide
derived from 4-amino-1,3-dihydroxy-2,6-dinitrobenzen); while your wrongly and ambiguous 4-diazo-2,6-dinitroresorcinol is in fact refering to
4-diazo-3-hydroxy-2,6-dinitrophenoxide (a para diazo-oxide derived from the very same 4-amino-1,3-dihydroxy-2,6-dinitrobenzen)
As I exposed by drawing:
From trinitroresorcinol (1,3-dihydroxy-2,4,6-trinitrobenzen) by monoreduction of a NO2 group you only get two possible isomeric
amino-dinitroresorcinol:
1°) the amino comes from reduction of the nitro trapped between te two NO2
--> 2-amino-1,3-dihydroxy-4,6-dinitrobenzen
2°) the amino comes from reduction of one of the two others NO2 that are next to one OH (in ortho) and in para of the other OH...and those only
provide one isomer by degenerescence linked to the symetry of the molecule (the axis being between the two OH)...so reducing the 4 or the 6 nitro will
always lead to 4-amino-1,3-dihydroxy-2,6-dinitrobenzen
From 1°) aka 2-amino-1,3-dihydroxy-4,6-dinitrobenzen
by diazotation you will get only one isomeric 2-diazo-oxide because the -N=N-OH may react (anhydrize) with the -OH in position 1 or with the -OH in
position 3 what is the same (again degenerescence) also because of the inter-OH molecular symetry...
So in fact:
2-diazo-(1-oxide)-3-hydroxy-4,6-dinitrobenzene
is equal to
2-diazo-3-hydroxy-4,6-dinitrophenoxide (not phenol because the OH in position 1 is not present anymore and phenoxide means implicitely that the oxide
is in position 1)
what is equal to
2-diazo-(3-oxide)-4,6-dinitrophenol (the OH in position 1 implicitely)
and if you flip along the free OH axis this is equal to
6-diazo-(5-oxide)-2,4-dinitrophenol (cf supra)
From 2°) aka 4-amino-1,3-dihydroxy-2,6-dinitrobenzen
by diazotation you will get only two isomeric 4-diazo-oxide because the -N=N-OH may react (anhydrize) with the -OH in position 1 (thus in para
position vs itself) or with the -OH in position 3 (what is ortho position vs itself)...
So in fact:
para case
4-diazo-(1-oxide)-3-hydroxy-2,6-dinitrobenzene
is equal to
4-diazo-3-hydroxy-2,6-dinitrophenoxide (not phenol because the OH in position 1 is not present anymore and phenoxide means implicitely that the oxide
is in position 1)
what is equal to
2-diazo-(5-oxide)-4,6-dinitrophenol (the OH in position 1 implicitely)
and if you flip along the free OH axis this is equal to
6-diazo-(3-oxide)-2,4-dinitrophenol (cf supra)
ortho case
4-diazo-(3-oxide)-1-hydroxy-2,6-dinitrobenzene
is equal to
4-diazo-(3-oxide)-2,6-dinitrophenol
what is equal by flipping along OH axis to
4-diazo-(5-oxide)-2,6-dinitrophenol
what is equal to
2-diazo-(1-oxide)-5-hydroxy-4,6-dinitrobenzene
what is equal to
2-diazo-5-hydroxy-4,6-dinitrophenoxide
what is is equal to
6-diazo-(1-oxide)-3-hydroxy-2,4-dinitrobenzene
or
6-diazo-3-hydroxy-2,4-dinitrophenoxide
Yes you start to feel dizzy and suffer from vertigo...your brain is overheating and fuming      and this
gives you a better view of the problem... and this
gives you a better view of the problem...
(*) So what you wrote implicitely is exactly what I wrote explicitely...
I deliberately noted the oxide place and number to make things clearer...because this is the main source of confusion...
I really think that everybody here should always do so...to be sure what we speak about.
(**) If there is little ambiguity (***) when dealing with DDNP beause the diazo can only form a single diazo-oxide
with the only OH present; when dealing with dihydroxyphenol (resorcinol)...confusion arises and speaking about DDNR becomes a problem.
In common usage for the future we should refer DDNP to be Diazo-Di-Nitro-Phenoxide and not Diazo-Di-Nitro-Phenol because the -OH is inexistant into
the molecule...for analogy it is like saying that CH3-O-CH3 is methyl-methanol...while it is an ether (dimethyl-ether).
(***)The tiny ambiguity may come from the various true isomers and from the effective numerotation/nomenclature used...
so a little molecular drawing is never too much.
[Edited on 22-2-2017 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
It is frustrating and confusing what is the nomenclature derived issue and trying to do the mental algebra to translate what is being described at one
time in history using an "original language" kind of nomenclature and associated ring position numbering, with the changed naming language of a
different time in history where the same compound is subjected to a different naming convention. Often the naming chosen to be used follows a pattern
that is an association with the trivial name for the precursor compound used in synthesis of a product compound that is then regarded as a derivative
"offspring" child of the "parent compound". The trivial name for the derivative or some acronym adopted is then not formally correct, as Louis
pointed out regarding DDNP which is not actually any longer a phenol but a phenol anhydride resulting from the diazo-oxide bridge that has displaced
the hydroxyl hydrogen of the parent phenol. Even so, DDNP will forever be called DDNP simply because every chemist knows the historical name even
though it is not precisely technically correct.
In common usage what is the compound DDNP is understood to be "the diazo compound of Griess who discovered and published the origin of diazo
chemistry",
so the acronym is not formally correct but is a historical trivial name for what is a compound every chemist knows as a "basic compound" no matter
what long name it may be given.
In my opinion it is likewise the case for the compound DDNR, which analogously is "the compound of Benedikt and Hubl" and is another historical
trivial name and acronym that has a historical basis that should be understood by every chemist what structure is DDNR, no matter what long name it
may be given.
The subject of this nomenclature issue was discussed earlier
http://www.sciencemadness.org/talk/viewthread.php?tid=439&am...
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
It is hard to believe there really exists a N=N-O bond for diazophenols:
1. The ring strain produced for pDDNP would be really immense for an aromatic and perhaps more importantly, much higher compared to oDDNP. Yet, the
difference in energy release and initiating properties between the two seems very similar. (Although the latter still needs proper verification, I
would say pDDNP is somewhat more energetic, but perhaps also seems so due to mono-nitro present)
2. One would expect that Tdec would be lower for pDDNP due to increased ring strain, while the opposite is true
3. In all likelyhood, both DDNP's dissolve in strong acids to form a diazonium salt
4. Even moderately strong nucleophiles can easily react with the diazogroup leading to substitution, even under (almost) waterfree conditions
5. No other compounds containing a R-N=N-O-R group have been found (fafaiaao), while diazotates are charged (behaving more like a OH- salt IIRC)
I think depending on conditions, DDNP's represent something between a zwitterionic and quinoid structure. In very strong concentrated acids it can
behave as the former, but the electrons are not completely sure where they should reside the most. 
So where does the extra energy for pDDNP come from in Klapotkes calculations? From assuming the NNO bridge?
[Edited on 24-2-2017 by nitro-genes]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
In the case of the p-DDNP the diazo-oxide bridge has only one path to form which is straight across the ring, since there is no other hydroxyl
available. And I think the nitros on each side of that para hydroxyl amplify the attraction and steer that diazo-oxide linkage formation there sort of
like magnets steering an electron beam. And I think the combined effect of those "steering groups" is stronger than the attraction of an adjacent
hydroxyl as a prospective target, particularly if that hydroxyl hasn't formed yet because it is a nitro at 3 and must decompose to a hydroxyl
subsequent to the formation of the para diazo-oxide linkage across the ring. I could be wrong, but that is my guess what is occurring. If the second
hydroxyl was already present before the diazo-oxide linkage formed, then it might preferentially form the linkage with the adjacent hydroxyl similarly
as is the case for o-DDNP. There is an uncertainty about these reactions and sequence that could make possible different isomers. To sort out what is
the whole true story would require performing different paths of synthesis and comparing the products to see which are the same and which may be
different.
I think the extra energy for the p-DDNP comes from the para diazo-oxide bond and added stability comes from the symmetry.
Thanks to Boffis for the file for the Klapotke article that is published. We have been discussing what was information in an early preview draft that
had not yet published so there may be unknown changes or corrections. Attached is the file for the article as published.
Attachment: 4-Diazo-2,6-dinitrophenol and 6-Diazo-3-hydroxy-2,4-dinitrophenol.pdf (432kB)
This file has been downloaded 567 times
I already see what I believe is a problem in the NMR data table figure description.
The first two compounds based on identification of structure diagrams appear to be transposed. The legend shows compound [7] as the middle compound,
but the structural diagram is for iso-DDNP which is compound [4]
Likewise for the first structural diagram shows compound [7] but the legend for the table says it should be compound [4].
So the table legend for Figure 3 is wrong for the first two compounds. I am pretty sure the top graph is for [7} and the middle graph is for [4].
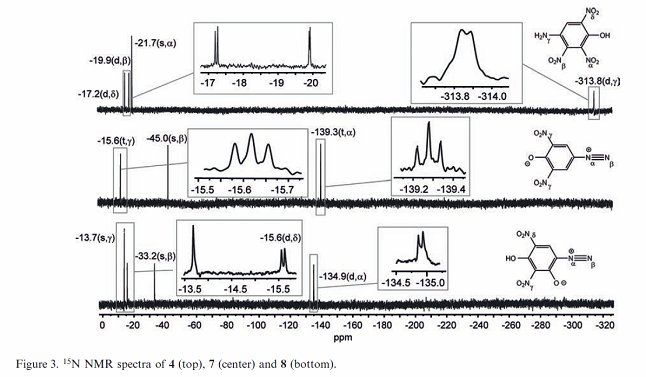
[Edited on 2/24/2017 by Rosco Bodine]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Okay I think I have sorted out what has been puzzling about this Klapotke article and why his reported compound [8] may differ from Meldola's p-DDNR
structurally with regards to the diazo-oxide appearing as an ortho linkage to the adjacent hydroxyl to the diazo, instead of appearing as a para
linkage diazo-oxide to the hydroxyl across the ring directly opposite the diazo.
If what Klapotke is reporting is accurate, (and I now believe it is), then it is possible to produce either isomer depending upon the method of
diazotization applied to the compound [7] which is 4-amino-2,3,6-trinitrophenol. Following the method of diazotization of Klapotke depends upon the
decomposition of the nitro at 3 to diazotize the amino, so that sequence makes available the ortho hydroxyl adjacent to the diazo as soon as it forms.
In that case, if that is what does occur then indeed HODDNP could be the result.
In an earlier post linked here Meldola used a different means of diazotization applied to the same 4-amino-2,3,6-trinitrophenol, and used small
portions of solid nitrite added to a solution of the 4-amino-2,3,6-trinitrophenol in cold H2SO4, under conditions where the 3 nitro remained intact as
the diazotization was
performed. In that circumstance there was only one hydroxyl available for the formation of the diazo-oxide bridge and that would be the para, just the
same scenario as occurs for p-DDNP.
http://www.sciencemadness.org/talk/viewthread.php?tid=439&am...
Meldola actually isolated the 4-diazo-2,3,6-trinitrophenol(1-anhydride) as a dense yellow microcrystalline powder precipitated from dilution of the
soluble diazonium sulfate. Boiling that material with a strong solution of sodium acetate decomposed the 3 nitro and produced the sodium salt of
p-DDNR, even though it is clear Meldola was not certain himself of the structure, and did not positively identify one isomer. Given the reaction
sequence as the most logical influence, it would be likely that Meldola was describing the para isomer variant different from the ortho diazo-oxide
DDNR of Bendikt and Hubl. The DDNR of Benedict and Hubl has the ortho hydroxyl already present, as would other diazotization schemes applied to
styphnamic acid. So evidently the ortho bridge for the diazo-oxide would be the usual, "normal" structure for DDNR and is the exact same compound as
Klapotke's HODDNP.
Klapotke's HODDNP = Benedikt and Hubl's DDNR
Meldola describes what would be an iso-DDNR or p-DDNR variant that has the same para diazo-oxide bridge as p-DDNP.
So the normal, more usual diazo-oxide bridge is the same ortho structure for DDNP and DDNR. The classical DDNP of Griess is o-DDNP and the classical
DDNR of Bendikt and Hubl is o-DDNR.
The para variant of DDNR would then be the para "iso" structure for the p-DDNP and for the p-DDNR. The enhanced energy and stability that appears for
p-DDNP would reasonably apply also for the p-DDNR.
So at this point, the logical hypothesis for me to make is that depending upon the method of diazotization applied to Klapotke's compound [7]
4-amino-2,3,6-trinitrophenol it is possible to obtain either isomer desired. Following the method of Klapotke the product will be HODDNP, o-DDNR, but
in the alternative, following the diazotization method of Meldola and hydrolyzing the product will be p-DDNR, or iso-DDNR, obtained as the sodium or
potassium salt, with the free acid p-DDNR obtained by acidification with HCl or other acid.
So I think ...the mystery is solved!!!! See how easy that was    
Hey it was so much fun ...let's do it again 
To summarize what has been distilled from the study of the available references, the nexus which I had identified for the compounds described by
Benedikt and Hubl, Von Herz GB207563,
Hagel and Redecker US4246052, and Klapotke compound [8]
appear to be all DDNR ....and about that nexus I was correct.
However, all of those examples of DDNR (referenced to ring positions for resorcinol) are the ortho structure for the diazo-oxide from what would be
position 4 of the diazo to the adjacent 3 position hydroxyl, made into a 3 anhydride.
The DDNR reported by those several authors is
4-diazo-2,6-dinitroresorcinol-(3-anhydride) o-DDNR = HODDNP
I was incorrect in my early impression that the "normal" DDNR diazo-oxide structure would be the para structure that would be from the diazo at 4 to
the hydroxyl at 1, forming a 1 anhydride.
That is the exceptional structure obtained by an alternate scheme for diazotization used by Meldola.
The p-DDNR of Meldola would be.
4-diazo-2,6-dinitroresorcinol-(1-anhydride) p-DDNR or iso-DDNR
The name is somewhat misleading for p-DDNR since the compound is not strictly a resorcinol "derivative" but is structurally similar.
That para diazo-oxide iso-DDNR or p-DDNR is the "special case" isomer obtained by a modified diazotization scheme of Meldola, applied to a precursor
that is Klapotke's compound [7].
Paracetamol is the starting material for p-DDNP and p-DDNR and each are expected to have enhanced properties over their more commonly known analogous
ortho isomers.
[Edited on 2/26/2017 by Rosco Bodine]
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
Why the reaction of trinitrophenol and cyanide occurs so readily to form isopurpuric acid is beyond me, but the product upon partial hydrolysis of one
of the nitrile groups and diazotization to the benzazimide seems OTC. "explodes violently at 210 deg C" sounded promising in any case  . .
Seems the reaction of nitrophenols with cyanides to purpuric acids was already discussed to some extent in this thread:
https://www.sciencemadness.org/whisper/viewthread.php?tid=10...
Though no mention of a diazogroup or benzazimide, so I therefore decided to post this in the DDNP thread. Technically, the compound contains a
diazogroup, two nitro groups and a hydroxyl, so posting this here seemed appropriate.
Haven't seen much on benzazimide derivatives as energetic materials before. Also curious what the reaction of cyanide and TNP would do when no excess
of cyanide is used. Interesting reaction, probably occuring via a meisenheimer complex due to basicity and nucleophilic attack by the cyanide ion like
in VNS, but if so why can't you directly add an azide group on TNP? The reduction of one of the nitrogroups is maybe helping here, but where did the
missing oxygen go, is cyanate maybe acting as the nucleophile here?
[Edited on 5-10-2017 by nitro-genes]
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Thank you Nitrogenes for that sharing...
Very strange molecule (those iso and meta-purpuric acids) and interesting reaction indeed... never read about it...seems so easy 
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
Previously I attempted the further nitration of isopicramic acid using KNO3/SA and obtained a small yield (<10%) of 2,3,6 trinitro 4-diazophenol
for which the 3-nitrogroup easily hydrolyzes producing 2,6 dinitro 4-diazo resorcinol. I was curious if using nearly anhydrous mixed acids and very
low temperatures to suppress oxidation as much as possible could produce a higher yield.
2 grams of isopicramic acid (0.01 moles) was dissolved in 30 grams freshly distilled azeotropic sulfuric (98.3%) at room temperature. The dissolution
itself was not only somewhat exothermic but also released some gasses. the clear orange solution was then put in the -23C freezer and when fully
cooled, 1.39 (0.022 moles) grams of ~98% nitric was added, briefly swirled and put back in the freezer. A small sample was taken, crashed on ice and
diazotized every day, the first few days, mainly p-DDNP was produced, indicating that nitration at these temperatures is VERY slow. After 5 days in
the freezer still large amounts of a light yellow compound were present (p-DDNP, 2,6 dinitrobenzoquinone, maybe the triazene Philou mentioned).
Reckoning the nitration would take a month or so or wouldn't proceed at all at these temperatures, I decided to led it further react for 24 hours at 4
deg. C. in the fridge. The colour had then changed to a dark red and small bubbles were visible in the mix. While in the icebath, the mixture was then
diluted dropwise with an icecold water/sodium nitrite solution to a total volume of 100 ml. Upon adding ethylacetate for the extraction, a pale yellow
compound precipitated at the interphase, which I suspect to be the 2,3,6 trinitro 4-diazophenol itself. (Maybe it would precipitate from the dilute
sulfuric as well with some more patience  ). Extractions were continued until all
precipitate was extracted. When all ethyl acetate had evaporated, 20 ml of distilled water was added and left to stir at room temperature for 1 hour,
the precipitate washed with another 10 ml cold water and dried. Yield was 0.37 grams of a light yellow crystaline solid, presumably 2,6 dinitro
4-diazo resorcinol. ). Extractions were continued until all
precipitate was extracted. When all ethyl acetate had evaporated, 20 ml of distilled water was added and left to stir at room temperature for 1 hour,
the precipitate washed with another 10 ml cold water and dried. Yield was 0.37 grams of a light yellow crystaline solid, presumably 2,6 dinitro
4-diazo resorcinol.
I tried to grow well defined 2,6 dinitro 4-diazo resorcinol crystals from slow evaporation of a MEK solution, but it seems to react with the solvent
itself, producing an orange coloured impurity. Similar to DDNP's, the best method of recrystallization seems from 65% nitric since this oxidizes most
impurities. 100 mg of crude 2,6 dinitro 4-diazo resorcinol was added to a 20 ml beaker and made into a paste with a few drops of water. Then 65%
nitric was added with slight warming untill everying dissolved (2 mls or so). Hot water was added to a total volume of 20 ml and put in the fridge for
several hours. The DDNR crystallizes as long bright yellow plates (attachment).

Attachment: 2,6-dinitro-4-diazoresorcinol - Copy.avi (2.8MB)
This file has been downloaded 1060 times
[Edited on 31-10-2017 by nitro-genes]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Have you tried forming the potassium or nickel or strontium or barium salt?
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
No, the salts are supposedly extremely sensitive to friction and can explode upon crystallization, so I never made more than a few mg's. Even when a
suspension of 2,6 dinitro 4-diazo resorcinol in water is stirred briefly with a neutral solution of KNO3, the potassium salt is already formed too
some extend and the resulting product makes DDT in sub-mg amounts, so care should be taken to avoid any contact with metals or salts.
I'm not entirely sure this is 2,6 dinitro 4-diazo resorcinol as well, the product I obtained seems more easily decomposed in hot water and not sure if
the reactivity towards MEK is also expected for the 2,6 dinitro 4-diazo resorcinol.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Sensitivity issues can sometimes be resolved by a specific compound, but I don't know what to suggest. Similar problems have occurred for many other
candidate initiators.
Perhaps an amine salt or a basic salt or double salt would reduce the sensitivity.
|
|
|
dave321
Harmless

Posts: 45
Registered: 22-11-2012
Member Is Offline
Mood: No Mood
|
|
the strontium salt can be co precipitated allowing the formation of strontium sulphate which I believe serves to reduce the sensitivity
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
Was mainly curious if the nitration of isopicramic could be improved somewhat, any ideas if/how this could be further improved?. Curious what exactly
causes the side occuring oxidation of isopicramic to the quinone imine, maybe some direct rearangment of a nitramine instead of hydrolysis, if so
would theoretically a stronger acid than sulfuric help prevent this instead of anhydrous conditions as I assumed previously?
Would be interesting to look at possible double salts etc, though accurately experimenting with sensitive salts of these compounds would be difficult
at a scale I would be comfortable with. Briefly touching it with a glowing splint seemed effective enough in desensitizing it completely 
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
One scheme that might be worth experiments would be to first form a basic styphnate and to then attempt conversion to a neutral complex salt which
would require 2 equivalents of the DDNR, one for each of the hydoxyls of a divalent metal basic styphnate. Similarly a basic picrate or a basic
nitrate or basic perchlorate might be converted to a neutral complex salt by 1 equivalent of DDNR. Any of the bivalent metals would be candidates for
experiments. Complexation involving ammonium salts would also seem possible as a means of reducing sensitivity.
Magnesium salt of styphnic acid is highly soluble and that may likewise be true for the Magnesium salt of DDNR.
Acetic anhydride is the key to simplifying everything by converting the paracetamol to the acetate before nitration.
[Edited on 11/4/2017 by Rosco Bodine]
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by nitro-genes  | No, the salts are supposedly extremely sensitive to friction and can explode upon crystallization, so I never made more than a few mg's. Even when a
suspension of 2,6 dinitro 4-diazo resorcinol in water is stirred briefly with a neutral solution of KNO3, the potassium salt is already formed too
some extend and the resulting product makes DDT in sub-mg amounts, so care should be taken to avoid any contact with metals or salts.
I'm not entirely sure this is 2,6 dinitro 4-diazo resorcinol as well, the product I obtained seems more easily decomposed in hot water and not sure if
the reactivity towards MEK is also expected for the 2,6 dinitro 4-diazo resorcinol. |
Diazoniums may react with phenols (diazo coupling reaction)... although I don't know if it will stil be reactive towards another phenol molecule when
the diazonium is present aswel with a OH into the same molecule and thus forming an intramolecular linkage like into DDNP or DDNR .
Would be nice to test your compounds with hydroxybenzene (phenol) and see if you get a new colorfull compound (diazo coupling usually provide
colourizers).
HO-C6(NO2)2-N=N-OH + C6H5-OH --> HO-C6(NO2)2-N=N-C6H5-OH + H2O
Why do I speark about this?
If this happens, then there is a chance aceton (propanone) or butanone (MEK - Methyl-Ethyl-Keton) reacts with the diazonium under their enol form...
CH3-CO-CH3 <--==> CH3-C(OH)=CH2
CH3-CH2-C(OH)=CH2 <==--> CH3-CH2-CO-CH3 <--==> CH3-CH=C(OH)-CH3
Phenol (ph-ENOL) as its name reveals it ... is a keton in disguise and it usually diazocouples in para or ortho position if para position is already
busy/occupied...
[Edited on 6-11-2017 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
DDNP forms a 1:1 low melting eutectic with picric acid when solvent acetone used to wet the mixture to a paste is evaporated with warming, and this
may involve a diazo coupling reaction. A similar effect may occur for DDNR and styphnic acid.
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
Thanks Philou for explaining the probable cause of the reactivity with butanone. I've used acetone for DDNP recrystallization regularly and never
really observed any reaction there, maybe the increased acidity of DDNR is contributing here, or maybe because acetone can form a terminal -ene in
contrast to butanone?
Regarding the ease of going from the O-acetylation of acetaminophen to DDNR: Taking "Synthesis and Energetic Properties of 4-Diazo-2,6-dinitrophenol
and 6-Diazo-3-hydroxy-2,4-dinitrophenol" as an estimate, the total yield would be something like: 0.81*0.56*0.76*0.75=26% overal yield. This is
probably biased by the small scale and focus on purity, though the nessecary 4-amino-2,3,6-trinitrophenol intermediate is not very stable and likely
dangerous to store. The nitration of acetaminophen can probably be optimized to 90+ yields with good temperature control, high purity acetaminophen
and a nitrous scavenger (like guanidine nitrate in a previously posted paper), so even a 50% yield (doubt this is possible though) for the nitration
of isopicramic to DDNR could be competitive with other published synthesis routes, perhaps even those from resorcinol/styphnic (mono reduction of
styphnic to styphnamic using anhydrous stannous chloride also only had a ~60% yield IIRC). The nitration of isopicramic is certainly the most
interesting route, since it has not been published or examined previously, besides, it is the only completely OTC route possible.
That is...if it really is DDNR...one of the things that strikes me is that the burn video of the pure compound isolated from the nitration of
isopicramic shows hardly any sooth, despite the fact that DDNR would only have a marginally better oxygen balance compared to DDNP. The presence of
the diazo group can be tested for probably, though another possible hydroxyl, the presence of an extra nitro group originating from a putative
tetranitro 4-aminophenol intermediate would be hard to test for, since an ortho dinitro presence would be just as suceptible to nucleophillic
replacement f.e as an diazogroup. If you assume however a tetranitro 4-diazophenol would actually exist, the diazogroup itself would likely be liable
for decomposition, resulting in a tetranitro -p-hydroquinone, or tetranitro/pentanitrophenol. This seems very unlikely though, since the initial
product of the nitration of isopicramic seems to produce NOx when reacted with water and not N2 gas, so this makes it unlikely that the product
obtained is derived from a putative tetranitro compound.
I have wondered if it would be possible for the 2,3,6-trinitro 4-aminophenol to rearrange differently, maybe resulting in a furoxan or furazan group.
Still wonder if DDNR would be able to form salts like is published, wouldn't this be hard to explain assuming a quinone or zwitter-ionic like
structure? It is interesting to notice that the difference in elementary composition between an ortho-diazoquinone and a furazan would be negligible.
Klapotke et al have found no furazan/furoxans though when heating 2,3,6 trinitro-4-aminophenol in 65% nitric. Besides, I doubt a furazan/furoxan
derivative would behave as energetic in the burn-test-video I previously posted.
All in all the only likely possibility is that the compound is DDNR after all, pfff glad I could convince myself again. 
[Edited on 11-11-2017 by nitro-genes]
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by Rosco Bodine  | | DDNP forms a 1:1 low melting eutectic with picric acid when solvent acetone used to wet the mixture to a paste is evaporated with warming, and this
may involve a diazo coupling reaction. A similar effect may occur for DDNR and styphnic acid. |
The main problem with TNP (picric acid) is that it forms also adducts (complexes by aromatic pi electron stacking) with a lot of different aromatic
rings... so this new compound will change the macroscopic properties of the mix (collogative properties like melting point).
So into that specific case, you may have a lot of possible reactions diazo transfert (bridging to another molecule), reaction with the solvent or
adduct formation...
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
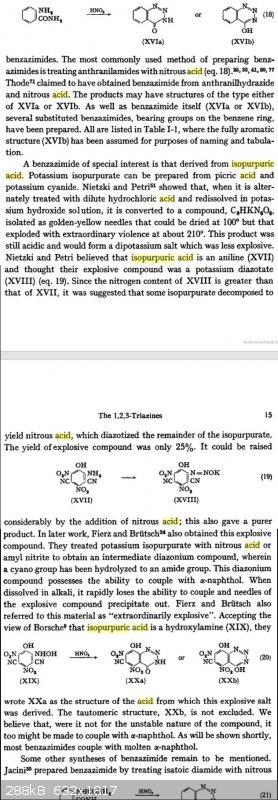
Found an article about the synthesis of isopurpuric acid from picric and the benzazimides derived from them by further reaction with nitrous acid.
Basically, the synthesis consists of reacting a reasonably dilute solution of picric acid with a large excess of potassium cyanide (seems very
dangerous, releasing large amounts of hydrogen cyanide probably) to obtain a supposed 80% yield of the isopurpuric acid. Would be interesting to
experiment with sometime, the salts of the resulting benzazimides are described as explosive even in minute amounts.
Attachment: php4gbXmg (466kB)
This file has been downloaded 497 times
|
|
|
nitro-genes
International Hazard
    
Posts: 1048
Registered: 5-4-2005
Member Is Offline
|
|
Had some DDNR left from the previous nitration of isopicramic, it showed some discolouration from exposure to indirect sunlight, so decided to do some
tests and be done with it. DDNR seems very soluble (even at room temperature) in a zinc acetate/acetic buffered solution at pH=6, so it appears the
zinc salt could be used to produce other salts if needed. Below pH of 8, N2 gas is slowly produced, forming a brown compound and deep red solution as
described earlier . Some NaN3 was also added to a cooled solution of the zinc salt, immediately leading to N2 production and upon standing a brown
gelatinous precipitate was produced that wasn't energetic. The decomposition temperature of DDNR seems similar to that of p-DDNP when together on the
hotplate, exploding at around 170-180 deg. C. Interestingly, both samples seemed to melt first, followed by explosion only seconds later. Together
with the dissolution in concentrated acids, it is very likely to be DDNR after all.
Also made a few mg's of the potassium salt of 2,6-dinitro 4-diazoresorcinol (attachment), by precipitation from the zinc salt using a solution of
potassium nitrate. It separates on strong cooling as yellow/orange triangular shaped plates that were incredibly static, jumping around under the
binocular when touched. Rough ignition temperature was determined on the hotplate, surprisingly, the explosion temperature was a lot higher than for
the acid DDNR itself, darkening at 150-160 deg C, explosion within 10 seconds at 215-225 deg C, immediate explosion at 240-250 deg C.

[Edited on 24-12-2017 by nitro-genes]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
Manganese is another possibility to consider for a salt or complex salt.
I have an idea that is pure speculation and may or may not be possible.
A compound that has been mentioned before in this thread is KDNBF.
Rathsburg described and patented an isomer that was called a dinitrodinitrosobenzene which is not a correct formal nomenclature for the class of
compounds which has several isomers.
I haven't looked too closely at this to form any well developed opinion whether the reaction is likely or not likely to work, to lead to a possible
phenolic isomer of KDNBF using isopicramic acid as the starting material, oxidized with household bleach, to a possible nitrobenzofuroxan
intermediate, subsequently further nitrated to a possible trinitrobenzofuroxan as might form a "KTNBF" (potassium salt), or the nitration might stop
at a dinitro isomer of KDNBF.
In an earlier post there was described a mono nitration of acetaminophen and deacetylation to the 2-nitro-4-aminophenol.
http://www.sciencemadness.org/talk/viewthread.php?tid=439&am...
This material would likewise be a candidate for experimental oxidation with household bleach and subsequent nitration.
The added hydroxyl of the phenol on the ring could possibly lead to phenolic derivative of KDNBF, if the hypochlorite oxidation of the
nitroaminophenol proves to be analogous to the known oxidation of the ordinary orthonitroaniline having no phenolic hydroxyl.
If the ortho relation of a nitro and amino is necessary for the oxidation by bleach to lead to the benzofuroxan, then the reaction would fail for
isopicramic acid, and picramic acid, but could work on the 3-nitro containing nitro derivative of paracetamol which has been further acetylated by
acetic anhydride to form acetaminophen acetate prior to nitration. It could work on other isomers where there is found an adjacent amino and nitro.
If an analogous oxidation with bleach and subsequent nitration does lead to a hydroxylated dinitrobenzofuroxan derivative, it would likely be bibasic
and could form an interesting copper II salt. If a trinitro derivative is the ultimate product from an isopicramic acid first oxidized and
subsequently nitrated, the energy of such a compound should exceed the energy of KDNBF.
I absolutely do not know if such an analogous reaction and synthetic path and resulting product/s is possible or not. The possibility is something
that seems to be a reasonable inference that may be drawn from the reactions described for KDNBF.
Attachment: 877237 Investigation of KDNBF DTIC document.pdf (3.4MB)
This file has been downloaded 932 times
Attachment: GB190844 salts of dinitrodinitrosobenzene.pdf (172kB)
This file has been downloaded 450 times
Attachment: Page B 45 from PATR Vol. 2 B-C.pdf (124kB)
This file has been downloaded 430 times
Attachment: Pages B 68-69 from PATR Vol. 2 B-C.pdf (232kB)
This file has been downloaded 431 times
Attachment: DTIC 065743 M. Baer Pilot Plant Preparation of KDNBF.pdf (971kB)
This file has been downloaded 604 times
Attachment: US20020143189 Potassium dinitro benzofuroxane and method of making same.pdf (180kB)
This file has been downloaded 452 times
[Edited on 12/29/2017 by Rosco Bodine]
|
|
|
| Pages:
1
..
25
26
27
28
29
..
33 |
|