| Pages:
1
2 |
niertap
Hazard to Self
 
Posts: 76
Registered: 5-8-2011
Member Is Offline
Mood: hyper-conjucated
|
|
It's not that expensive to purchase over the internet, if you are more interested in the compound than the synthesis. It would likely not be cost
effective to make.
In the states you can only buy it as a bag of powder. It's not a naturally occurring compound, so it can't be called a supplement and is technically
a drug. It's available in Europe though. They also use picalomin(sp?) which is GABA fused with niacin. It apparently crosses the BBB somewhat,
then hydrolyzes. It's used frequently in russia as an anoxylitic.
To avoid the burned out feeling I suggest ribose, gatorade, acetyl-carnitine, vit. C, and CoQ 10. The antioxidants help ameliorate the oxidative
stress, ribose helps with energy formation(ATP), and the acetyl-carnitine is a proneurotransmitter that crosses the BBB. Piracetam can give you(me)
a headache if taken without the acetyl-carnitine.
Fyi piracetam is one of the most bitter things I have tasted. Almost as bad as the migraine medacine imitrex.
The effects are good, but hard to "put your finger on" It's mainly an overall increased mood, ability to think, and energy. I believe its mode
of action is from increasing blood flow in and to the brain.
Ignorance is bliss
Outliers in life are modeled by chemical kinetics
|
|
|
Lithium
Hazard to Others
  
Posts: 103
Registered: 25-2-2012
Location: Australia
Member Is Offline
Mood: Thinking!
|
|
Sorry for bringing up a relatively dead thread, but i have found a site that describes multiple ways to synthesise Aniracetam:
http://www.chemdrug.com/databases/8_0_hrigqxxtjyijmcqk.html
If one were not as lazy as i, they might search the database for other nootropics:
http://www.chemdrug.com/databases/db_8_3.html
Li
|
|
|
Hilski
Hazard to Others
  
Posts: 197
Registered: 13-9-2006
Member Is Offline
Mood: No Mood
|
|
Noopept is described as being exponentially stronger than the racetams (1000x stronger than Piracetam, for example) and it's widely available. So,
unless one wants to perform the synthesis' for the sake of the experience, there aren't a lot of good reasons to go to the trouble.
http://www.amazon.com/Noopept-Powder-000mg-Gram-Strongest/dp...
\"They that can give up essential liberty
to obtain a little temporary safety
deserve neither liberty nor safety. \"
- Benjamin Franklin
|
|
|
sigma742
Harmless

Posts: 5
Registered: 23-10-2012
Location: The excorcibsum
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by kmno4  | "Piracetam technology"
- paper found some time ago, possibly on this forum (but now I cannot find it), available thanks to some Damned User.
It can be useful and informative study about piracetam. |
If you read the paper the team proposes the use of glycine (common amino acid etc) and GLB - gamma butyrolactone... a precursor for GHB.
hah
|
|
|
Mildronate
Hazard to Others
  
Posts: 428
Registered: 12-9-2009
Member Is Offline
Mood: Ruido sintetico
|
|
[/rquote]
Don't even try with Sigma, you have to be a university or a major lab just to get their catalogue.
[/rquote]
Bullshit I have catalogue 
|
|
|
ChemistryGhost
Hazard to Others
  
Posts: 113
Registered: 5-7-2012
Member Is Offline
Mood: Supercooled 
|
|
What about the newer nootropics like dimethylamylamine, sunifiram, phenylpiracetam, NSI-189, PWZ-029 and others? The PWZ-029 would be interesting to
synthesize.
I'm so cool I'm ...
supercool! 
I like my drinks ... magnetically stirred! 
"Imagination is more important than knowledge" ~Einstein
|
|
|
smaerd
International Hazard
    
Posts: 1262
Registered: 23-1-2010
Member Is Offline
Mood: hmm...
|
|
I was trying to figure out an OTC way to the lactam precursor(2-Pyrrolidone) in aniracetam. Not that synthesizing aniracetam at home is cost
effective, but it seemed like a fun project. Unfortunately I found out about mid-way through a lot of my intermediates molecules were illicit
narcotics(GHB and analogs)... Found that to be a bit obnoxious to say the least. So I had to take a few steps back and think of a legal route. If I
find my notes I will share them, granted I haven't performed the synthesis' required yet but it could be a good launch pad for someone preparing to do
so...
Unfortunately I have a nuerological/psychiatric disorder that does qualify as having abnormal glutaminergic,dopaminergic, serotonergic, etc, pathways.
Fortunately with treatment of infrequent nootropics I have found a lot of peace of mind and was able to ween off of some far more dangerous and
side-effect prone medications. Granted I also found that taking nootropics too frequently doesn't seem to be as effective for me personally as
intermittent usage. Your mileage may vary.
Personally I have taken an interest in coluracetam (http://en.wikipedia.org/wiki/Coluracetam). Talk about a synthesis though...
|
|
|
ChemistryGhost
Hazard to Others
  
Posts: 113
Registered: 5-7-2012
Member Is Offline
Mood: Supercooled 
|
|
This might be one way of synthesizing PWZ-029. I'm not sure though.

[Edited on 2-10-2014 by ChemistryGhost]
"Imagination is more important than knowledge" ~Einstein
|
|
|
halogen
Hazard to Others
  
Posts: 372
Registered: 18-4-2004
Member Is Offline
Mood: No Mood
|
|
The pyrolysis of the common polymer PVP gives high yields of N-vinyl pyrrolidinone according to S. Moldoveanu. If that can be purified and oxidized -
peracid, epoxide to aldehyde?
[Edited on 2-10-2014 by halogen]
F. de Lalande and M. Prud'homme showed that a mixture of boric oxide and sodium chloride is decomposed in a stream of dry air or oxygen at a red heat
with the evolution of chlorine.
|
|
|
Metacelsus
International Hazard
    
Posts: 2531
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
Wouldn't epoxide opening give a diol and not an aldehyde? A Wacker oxidation is the only direct way I see to piracetam.
Alternatively, you could hydrolyze it to the plain pyrrolidinone, and then synthesize it from there.
|
|
|
halogen
Hazard to Others
  
Posts: 372
Registered: 18-4-2004
Member Is Offline
Mood: No Mood
|
|
No, they can rearrange. Acid catalyst, carbocation, hydrogen migrates.
F. de Lalande and M. Prud'homme showed that a mixture of boric oxide and sodium chloride is decomposed in a stream of dry air or oxygen at a red heat
with the evolution of chlorine.
|
|
|
ChemistryGhost
Hazard to Others
  
Posts: 113
Registered: 5-7-2012
Member Is Offline
Mood: Supercooled 
|
|
Quote: Originally posted by halogen  | The pyrolysis of the common polymer PVP gives high yields of N-vinyl pyrrolidinone according to S. Moldoveanu. If that can be purified and oxidized -
peracid, epoxide to aldehyde?
[Edited on 2-10-2014 by halogen] |
You could ozonate it instead to get the shorter aldehyde, then oxidize that to the carboxylic acid, then react with anisole using Chlorosulfuric acid
to get aniracetam. I assume so.
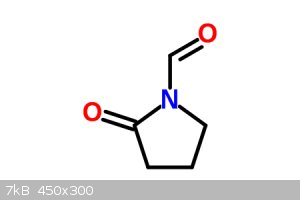

[Edited on 3-10-2014 by ChemistryGhost]
"Imagination is more important than knowledge" ~Einstein
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Sigh.
Whenever a New drug is synthesised, the Best the Law can do is List it, and prohibit the sale of the precursors.
Eventually Water will be classified as 'A Precursor For Drug Synthesis'.
The point is that despite current legislation, openly discussing what the Feds will be looking for Next Year isn't really that groovy.
Interesting, yet ultimately damaging to SM, as it is Public.
At the very least, move this stuff to a members-only section.
After all, it's an exercise in getting High, either thru direct use, or the spending of the money that ensues from the currently Legal drug sales if a
way to synth is found.
|
|
|
smaerd
International Hazard
    
Posts: 1262
Registered: 23-1-2010
Member Is Offline
Mood: hmm...
|
|
@ChemistryGhost - I notice a small error in your synthetic scheme. Didn't check all of it but I think you meant methylamine rather then ammonia for
the ring closing condensation.
@Aga - I really doubt any federal agency will be 'cracking down' on things like Aniracetam or Piracetam. They are not 'abusable', many people don't
see much of a difference between baseline. Little if not no 'recreational' value. If it did become a scheduled substance and was regulated by the FDA
I'd drive for 15 minutes ask my old doctor for a prescription and be set hah. She had seen first-hand the improvements I made by implementing it in my
life.
@Halogen - That's a great idea. I was eye-ing PVP back when I was interested in this. I'll try to find the paper you're referring too because that
would be infinitely easier than the route I was going.
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by ChemistryGhost  |
You could ozonate it instead to get the shorter aldehyde, then oxidize that to the carboxylic acid, then react with anisole using Chlorosulfuric acid
to get aniracetam. I assume so.

|
but from where would you get ozone other than producing it electrically(which i assume would be diificult) as the chemical methods of making ozone are
not efficient
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Tungsten.  |
That isn't how your receptors treat drugs. What would most likely result is a strong psychedelic because the amine is being protected by the cyclic
amide. This would make the compound less open to being broken down by mono amine oxidase and thus, more potent. |
i remember reading somewhere(maybe in psychedelic chemistry,or psychedelic encyclopedia or in strike's total synthesis) that if you go on substituting
the nitrogen then the potency of the drug would decrease but your logic that it would be protected from the mono amine oxidase enzyme also maked
sense
so i am a little confused now
|
|
|
ChemistryGhost
Hazard to Others
  
Posts: 113
Registered: 5-7-2012
Member Is Offline
Mood: Supercooled 
|
|
Flodafinil(Flmodafinil. CRL-40,940) is the successor to modafinil. Flodafinil is 4 times as potent as a eugeroic(wakefulness enhancer).
4,4'-Difluorobenzophenone might be a starting point for synthesis. In your face, Modafinil! Modafinil is wimpy wimpy wimpy. Flodafinil is hefty hefty
hefty. 
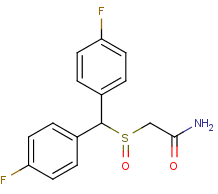
Flmodafinil
[Edited on 8-6-2015 by ChemistryGhost]
"Imagination is more important than knowledge" ~Einstein
|
|
|
ChemistryGhost
Hazard to Others
  
Posts: 113
Registered: 5-7-2012
Member Is Offline
Mood: Supercooled 
|
|
Sunifiram synthesis might require something fancy like propanoylpiperazine.
Bretazenil could also be interesting. Excess anxiety can block cognitive performance. Bretazenil seems pretty safe(or at least a base for which to
make a better anxiolytic and a much
much less toxic alternative to ethanol).
Also, 2C-D-5EtO and 2C-D-2EtO has far less psychedelic effects, but increased nootropic potency. Through more studies are needed and also there might
be a few unknown side effects.

Bretazenil.
Though this suggests that while moderate to severe activation of 5HT2a receptors cause hallucinations, mild activation enhances memory and problem
solving skills.
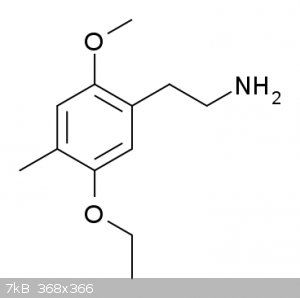
2C-D-2-EtO, or 1-(2-methoxy-4-methyl-5-ethoxyphenyl)-2-aminoethane.
[Edited on 8-6-2015 by ChemistryGhost]
"Imagination is more important than knowledge" ~Einstein
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by halogen  | | The pyrolysis of the common polymer PVP gives high yields of N-vinyl pyrrolidinone according to S. Moldoveanu |
won't a willgerot-kindler on the N-vinyl pyrrolidinone give piracetam ?
how would a wacker help? you will just end up with a ketone instead of the vinyl. How will you convert that to amide ? Quote: Originally posted by smaerd  | I was trying to figure out an OTC way to the lactam precursor(2-Pyrrolidone) in aniracetam. Not that synthesizing aniracetam at home is cost
effective, but it seemed like a fun project. Unfortunately I found out about mid-way through a lot of my intermediates molecules were illicit
narcotics(GHB and analogs)... Found that to be a bit obnoxious to say the least. So I had to take a few steps back and think of a legal route. If I
find my notes I will share them, granted I haven't performed the synthesis' required yet but it could be a good launch pad for someone preparing to do
so...
|
this might be a good method
http://www.google.co.in/patents/US3080377
|
|
|
Chemosynthesis
International Hazard
    
Posts: 1071
Registered: 26-9-2013
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by CuReUS  | Quote: Originally posted by Tungsten.  |
That isn't how your receptors treat drugs. What would most likely result is a strong psychedelic because the amine is being protected by the cyclic
amide. This would make the compound less open to being broken down by mono amine oxidase and thus, more potent. |
i remember reading somewhere(maybe in psychedelic chemistry,or psychedelic encyclopedia or in strike's total synthesis) that if you go on substituting
the nitrogen then the potency of the drug would decrease but your logic that it would be protected from the mono amine oxidase enzyme also maked
sense
so i am a little confused now |
Without getting into the actual binding affinities, these are two entirely separate charasteristics. The general pharmacodynamic presumption is target
binding affinity is proportional with potency. Unless you are targeting a metabolic enzyme, the potency of effect is separate from pharmacokinetics
influencing the half-life of the drug. You can have drugs that are less potent with a diminished elimination half-life (chemical or functional), vice
versa, or any combination thereof for a number of physicochemical or metabolic reasons.
This also differs with pharmacokinetic tolerance, as enzyme induction or inhibition may occur to shift metabolism towards hydroxylation and other
pathways such as COMT rather than a CYP dealkylation or the like.
The theory of combining drug action has been tried (rather infrequently and not with much success, relatively) with metabolically labile linkers, and
could potentially be utilized by diazotized compounds metabolized by gut fauna if oral administration were desired, but this is purely hypothetical
and more of a dual pro-drug approach (one not often utilized) which will see somewhat staggered absorption and compartmentalizations.
|
|
|
| Pages:
1
2 |