sterckxke
Harmless

Posts: 1
Registered: 21-11-2005
Location: Kasterlee
Member Is Offline
Mood: Xplosif
|
|
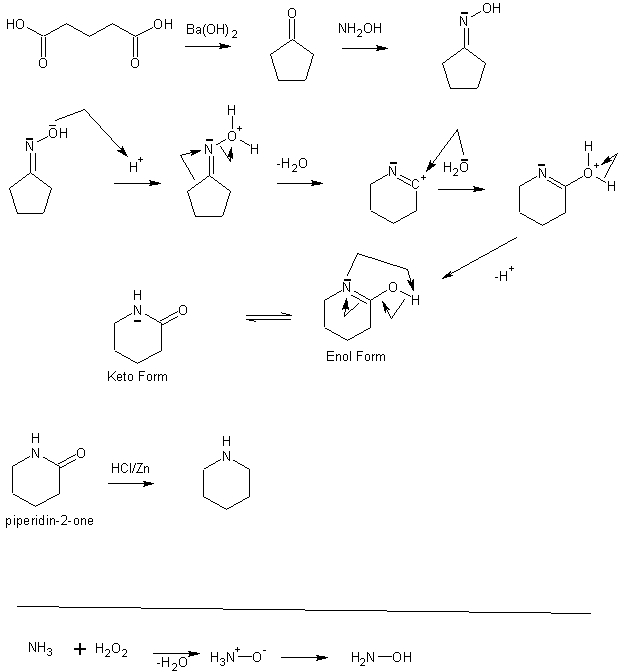
Synthesis of piperdine
well i was thinking about making piperdine myself(for use in another synth ) )
i read about the hoffman rearrangement and tought it could be usefull.So i found a route that starts from adipic acid(or cyclopentanon,if u have it)
it goes like:
HOOC-CH2-CH2-CH2-CH5-COOH --Ba(OH)2-->Cyclopentanon
Then: cyclopentanon + NH2OH -->cyclopentanon Oxime
now the beckmann rearrangement under influence of H2SO4(or PCl5 ) )
u get piperdin-2-one
reduce that with HCl/zn é Voila...
but the only problem i would think of would be the NH2OH(hydroxylamine)
then i read about amine oxides and the cope elimination reaction.Witch goes like this:
(3R)N + H2O2 -->(3R)N+O- --->(2R)NOH +alkene
But i thougt if i could substitute the R for a H
that wuld get u:
NH3 + H2O2 -->H3N+O- --->NH2OH
What do you think of this reaction?is it possible?High Yielding,....
comments plzz
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
How exactly does Ba(OH)2 reduce a dicarboxylic acid to a mono-ketone?
Tim
|
|
|
stygian
Hazard to Others
  
Posts: 242
Registered: 19-9-2004
Member Is Offline
Mood: No Mood
|
|
By dry distillation. Similar to calcium acetate-acetone. Only with dicarboxylic acids it forms cyclic ketones.
|
|
|
CherrieBaby
Hazard to Self
 
Posts: 91
Registered: 4-3-2005
Location: London
Member Is Offline
Mood: No Mood
|
|
These are all taken from "Organic Chemistry", Bernthsen, Revised by Sudborough, 1941 edition, Blackie & Sons.
1. When pentamethylene-diamine hydrochloride is strongly heated it yields piperidine.
NH2-CH2-CH2-CH2-CH2-CH2-NH2.HCl --> piperidine
2. The elimination of HCl from 5-chloroamylamine, (CH2Cl-(CH2)3-CH2-NH2. This elimination occurs when an aqueous solution of the base is heated on the
water bath; ring formation takes place, and piperidine HCl is formed.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by sterckxke
u get piperdin-2-one
reduce that with HCl/zn é Voila...
|
Yeah right! Since when can you reduce amides with Zn/HCl?
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
CherrieBaby
Hazard to Self
 
Posts: 91
Registered: 4-3-2005
Location: London
Member Is Offline
Mood: No Mood
|
|
How about piperidine via the decarboxylation of Pipecolic acid (which is alpha-carboxy-piperidine).
Pipecolic acid can be made from lysine.
L-Pipecolic acid from L-lysine; Bull. Chem. Soc. Jpn. 48, 1341 (1975).
DL-Pipecolic acid from DL-lysine; JOC 55, 738 (1990)
|
|
|
Drunkguy
Hazard to Others
  
Posts: 172
Registered: 23-12-2005
Member Is Offline
Mood: somewhat pissed.
|
|
For all the pain of the above procedure (Beckmann degradation - it's been a while!), did u guys not know that pyridine can be hydrogenated to
piperidine. I can understand that this is not easy to do though due to the fact that it has to be done under high pressure if using an ordinary
catalyst such as Pt(C). Also the fact that the catalyst will get poisoned if the source of hydrogen is not squeeky clean is another barrier. The
synthesis of diethylamine would not be as difficult though but I guess that piperidine is what we are really shooting for.
|
|
|
runlabrun
Hazard to Others
  
Posts: 172
Registered: 4-12-2004
Member Is Offline
Mood: No Mood
|
|
Not to mention the difficulty in obtaining pyridine these days.
Piperidine can be synthesised in easier ways such as those mentioned rather than the method in the original post.
-rlr
|
|
|
NERV
Hazard to Others
  
Posts: 152
Registered: 22-9-2002
Location: USA
Member Is Offline
Mood: Fluorinated
|
|
IIRC, piperidine can be had from the hydrolysis of piperine (1-piperylpiperidine) with a strong hydroxide. I am not sure of the full details as it was
just some random tid bit of information I came across one time, but I do know that piperine can be extracted in good yield from pepper.
Vir sapit qui pauca loquitur.
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
If one decides to go with the pepper extraction it was covered in this thread here: https://sciencemadness.org/talk/viewthread.php?tid=4698
|
|
|
Esplosivo
Hazard to Others
  
Posts: 491
Registered: 7-2-2004
Location: Mediterranean
Member Is Offline
Mood: Quantized
|
|
Correct me if I'm wrong, but what Tacho seems to have isolated is piperidine not piperine, which as you know are quite different. Piperine extractions
are usually, AFAIK, carried out by either using ethanol, methanol or DCM, but when piperidine is desired an alcoholic solution of KOH or some other
strong base is used. Am I right in concluding this, because I think there could really be some misunderstanding because of the relative similarity of
these two 'archaic' names (don't get me wrong, I love archaic nomenclature).
Theory guides, experiment decides.
|
|
|
S.C. Wack
bibliomaster
    
Posts: 2419
Registered: 7-5-2004
Location: Cornworld, Central USA
Member Is Offline
Mood: Enhanced
|
|
OK. Base is added in some procedures as there are supposedly acids that you dont want to extract.
It takes some time refluxing in 10% alcoholic KOH to get piperidine and piperate.
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
Yeah, I realize that piperidine and piperine are different, and that the above thread is the extraction of piperine. I meerly posted it as a way to
get piperine as an intermediate for the synthesis of piperidine.
Explosivo, you must mean that Tacho extracted piperine from pepper, not piperidine, right?
Piperine:

Piperidine:

|
|
|
Esplosivo
Hazard to Others
  
Posts: 491
Registered: 7-2-2004
Location: Mediterranean
Member Is Offline
Mood: Quantized
|
|
Its just that once I remeber reading that piperidine can be made from piperine by refluxing in a strongly basic alcoholic soln. I though that Tacho,
by refluxing the pepper with a strong base would most probably have extracted piperidine rather than piperine. I now realized that piperidine is a
liquid and Tacho's extract is infact solid, therefore I was proven incorrect. I tried the piperine extraction once but resulted in a horrendously
small yield. I will try it again, with DCM and a soxhlet soon.
Theory guides, experiment decides.
|
|
|
Drunkguy
Hazard to Others
  
Posts: 172
Registered: 23-12-2005
Member Is Offline
Mood: somewhat pissed.
|
|
There are synthetic details for the procedure online (if u look in the right place). It did not impress on me that the piperine hydrolysis was useful
for obtaining piperidine but was more geared toward iolating the 3,4-MD-benzoic acid
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
Hmm if I ever end up making an ozone generator, piperine could be interesting to do an ozonlysis with, Could get glyoxal  , and some other less interesting aldehydes. , and some other less interesting aldehydes.
Ozone is not the only thing that could do this IIRC, permanganate solution followed by periodic acid or lead tetraacetate could give the glyoxal as
well.
(hehe HNIW from pepper... ..Now thats madscience...) ..Now thats madscience...)
If extracting piperine from pepper with alcoholic K2CO3, would there be much reaction of the piperine to give piperidine as K2CO3 is a weak base?
[Edited on 27-1-2006 by rogue chemist]
[Edited on 27-1-2006 by rogue chemist]
|
|
|