subsecret
Hazard to Others
  
Posts: 424
Registered: 8-6-2013
Location: NW SC, USA
Member Is Offline
Mood: Human Sadness - Julian Casablancas & the Voidz
|
|
Distillation of Gasoline
Would distilling ethanol-free gas be a potential route to organic compounds like pentanes and hexanes? Before I start building a large scale gasoline
still, I'd like to know if you think this would be feasible, seeing as there are many compounds with close boiling points. After a preliminary
distillation, of course, the wide fractions from the large still would be taken to the lab and re-distilled.
Personally, I doubt that this will be feasible for separation, even with my ~3ft copper column.
[Edited on 26-11-2014 by Awesomeness]
Fear is what you get when caution wasn't enough.
|
|
|
Little_Ghost_again
National Hazard
   
Posts: 985
Registered: 16-9-2014
Member Is Offline
Mood: Baffled
|
|
I have a Gas Chromatograph from gasoline that came as a test wih the machine, there looks to be be plenty of stuff in there like hexane etc etc, with
a decent column you should be able to distill a good portion of it, the peaks were pretty well spread, so where a bit close together. I cant post it
at the moment as that pc isnt online.
Dont ask me, I only know enough to be dangerous
|
|
|
BromicAcid
International Hazard
    
Posts: 3227
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
Gasoline composition changes by the season and possibly even the month of the year. Additionally although the refineries send out the same gasoline
mixture to various gas stations, the distributors add their own proprietary mix of compounds advertised to clean the injectors and such. My gut tells
me you won't be able to get separation into specific hexane, hepane, toluene, etc fractions but you will be able to fraction to some extent and
collect. I.e., you will have a 35-50C fraction,50-60C fraction or whatever endpoint you decide for each fraction. Then the use of which would come
down to your own trial and error. Similar to the various grades of petroleum ether. Some fractions may contain unsaturated compound that begin to
polymerise, some might contain the various additives for octane rating such as MTBE, others might be chock full of aromatics (although according to
Wiki some countries limit aromatics). Anyway, found this too on one of the first google pages listing the composition of gasoline in one instance
showing isopentane, 2,3-dimethylbutane, n-butane, and n-pentane to be some of the most abundant components:
http://bcn.boulder.co.us/basin/waterworks/gasolinecomp.pdf
|
|
|
subsecret
Hazard to Others
  
Posts: 424
Registered: 8-6-2013
Location: NW SC, USA
Member Is Offline
Mood: Human Sadness - Julian Casablancas & the Voidz
|
|
Thanks for the advice.
Fear is what you get when caution wasn't enough.
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
I discussed it in the past on the forum. It would be nice to have different fractions, it might be possible to collect something useful. The extremas
would also pose useful compounds. For example, running the gasoline at a low temperature might carry over distinct, low boiling compounds. For
example, butane, and pentane may come over a still in rather pure form if you can keep the temperature low.
Another idea is the chlorination (or just halogenation) of unsaturated compounds, which would raise their boiling points. If aromatics existed in the
gasoline, or even just alkenes and alkynes, one could toss in some elemental iodine, and distill. Since such iodine compounds would have a much higher
boiling point than the rest of the gasoline, they could at least be separated from the rest of the gasoline.
|
|
|
careysub
International Hazard
    
Posts: 1339
Registered: 4-8-2014
Location: Coastal Sage Scrub Biome
Member Is Offline
Mood: Lowest quantum state
|
|
The remarks about gasoline variability are well taken, but it is also useful to take a gander at a detailed breakdown of the composition of a
"typical" gasoline sample (see attached).
Although, sure, the composition is variable - but the hydrocarbons in the gasoline are strongly controlled by a couple of important physical factors:
volatility and the octane number. There are also the common properties of industry wide cracking technologies which influence the presence of
different molecular species.
In other words - I would expect the variations to be predominantly in the relative prevalence of the molecules in the table rather than a
significantly different list of players.
It would be interesting to annotate this table with BP data and organize it into fractions.
If I were making my own petroleum ether, I would think about doing it through vacuum distillation at room temperature. Pet ethers boil at 20 C around
half an atmosphere, so the pressure reduction requirement is very modest and I would use an aspirator. Ice and salt as a receiver coolant? There would
be nothing electrical involved (except for a fume hood fan, though you might do this outside). Any sturdy gasoline-resistant plastic container could
be the feed flask so you could process several gallons at once.
Here in California I would want to use winter gas, and probably would treat it with CaCl2 to remove ethanol (and water) first.
After stripping it of its low boiling fractions, it could be mixed with regular gasoline and burned normally.
And after doing some reading about gas blending I find that blenders are using the same six or so feed stocks (e.g. fluid catalytic cracking light and
heavy fractions, straight-run gasoline, butane, etc.) but in different proportions.
Attachment: gasolinecomp.pdf (96kB)
This file has been downloaded 545 times
[Edited on 26-11-2014 by careysub]
[Edited on 26-11-2014 by careysub]
|
|
|
macckone
International Hazard
    
Posts: 2159
Registered: 1-3-2013
Location: Over a mile high
Member Is Offline
Mood: Electrical
|
|
Separating gasoline fractions is going to be tedious.
Varying pressure can help as can freeze crystallization.
Some fractions are may require chemical reactions
to clean up. Toluene and xylenes are probably going to be
easiest to extract and clean up.
|
|
|
Little_Ghost_again
National Hazard
   
Posts: 985
Registered: 16-9-2014
Member Is Offline
Mood: Baffled
|
|
Not sure how this will turn out, this is a GC taken of some petrol, I dont know how to change the formats so I just did a screen shot
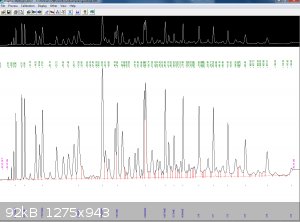
Dont ask me, I only know enough to be dangerous
|
|
|
Little_Ghost_again
National Hazard
   
Posts: 985
Registered: 16-9-2014
Member Is Offline
Mood: Baffled
|
|
I coudnt find the window screen that shows the window for peak settings, some of them do however overlap, so the suggestions of distilling over a
temperature range seem best.
Most of the over lap where it occurs is from benzene onwards.
Dont ask me, I only know enough to be dangerous
|
|
|
macckone
International Hazard
    
Posts: 2159
Registered: 1-3-2013
Location: Over a mile high
Member Is Offline
Mood: Electrical
|
|
Is this an actual GC or a sample profile from a library.
I ask because benzene peak should not be that
prominent in modern gasoline blends in a developed
country. Most countries limit benzene to fraction
of a percent.
|
|
|
Little_Ghost_again
National Hazard
   
Posts: 985
Registered: 16-9-2014
Member Is Offline
Mood: Baffled
|
|
Quote: Originally posted by macckone  | Is this an actual GC or a sample profile from a library.
I ask because benzene peak should not be that
prominent in modern gasoline blends in a developed
country. Most countries limit benzene to fraction
of a percent. |
That is from stuff sat in a garage for two years
but was done not that long ago, its in the examples folder because the guy that set the system up did a whole range for me, the software is for a
elmer perkins but the machine is actualy a HP
Dont ask me, I only know enough to be dangerous
|
|
|
Little_Ghost_again
National Hazard
   
Posts: 985
Registered: 16-9-2014
Member Is Offline
Mood: Baffled
|
|
I dont have the hydrogen yet so I cant rerun it for another week or so, but as soon as I get the hydrogen cylinder I will rerun with new fuel and fuel
from another petrol station/gsoline
Wait until you see the bio diesel one gave me a shock  we have stopped buying it
now from that supplier we have stopped buying it
now from that supplier
[Edited on 27-11-2014 by Little_Ghost_again]
Dont ask me, I only know enough to be dangerous
|
|
|
subsecret
Hazard to Others
  
Posts: 424
Registered: 8-6-2013
Location: NW SC, USA
Member Is Offline
Mood: Human Sadness - Julian Casablancas & the Voidz
|
|
The compounds in the document posted earlier are arranged by increasing BP.
Fear is what you get when caution wasn't enough.
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Quote: Originally posted by Little_Ghost_again  | I dont have the hydrogen yet so I cant rerun it for another week or so, but as soon as I get the hydrogen cylinder I will rerun with new fuel and fuel
from another petrol station/gsoline
Wait until you see the bio diesel one gave me a shock  we have stopped buying it
now from that supplier we have stopped buying it
now from that supplier
[Edited on 27-11-2014 by Little_Ghost_again] |
Now that sounds intriguing... now I can't wait to see the biodiesel chromatograph!
|
|
|
vmelkon
National Hazard
   
Posts: 669
Registered: 25-11-2011
Location: Canada
Member Is Offline
Mood: autoerotic asphyxiation
|
|
Quote: Originally posted by Little_Ghost_again  | Quote: Originally posted by macckone  | Is this an actual GC or a sample profile from a library.
I ask because benzene peak should not be that
prominent in modern gasoline blends in a developed
country. Most countries limit benzene to fraction
of a percent. |
That is from stuff sat in a garage for two years
but was done not that long ago, its in the examples folder because the guy that set the system up did a whole range for me, the software is for a
elmer perkins but the machine is actualy a HP |
You have a GC at home?
Signature ==== Is this my youtube page? https://www.youtube.com/watch?v=tA5PYtul5aU
We must attach the electrodes of knowledge to the nipples of ignorance and give a few good jolts.
Yes my evolutionary friends. We are all homos here. |
|
|
Little_Ghost_again
National Hazard
   
Posts: 985
Registered: 16-9-2014
Member Is Offline
Mood: Baffled
|
|
Quote: Originally posted by vmelkon  | Quote: Originally posted by Little_Ghost_again  | Quote: Originally posted by macckone  | Is this an actual GC or a sample profile from a library.
I ask because benzene peak should not be that
prominent in modern gasoline blends in a developed
country. Most countries limit benzene to fraction
of a percent. |
That is from stuff sat in a garage for two years
but was done not that long ago, its in the examples folder because the guy that set the system up did a whole range for me, the software is for a
elmer perkins but the machine is actualy a HP |
You have a GC at home? |
Yes I have just got a HP GC system, I am waiting for BOC to deliver the bottled gasses, I went to pick the system up from the place it was calibrated
and serviced, because it came with a control system and pc etc the Guy there gave me a couple of hours training on it, so I took along various
samples, mostly oils.
I have 3 different columns for it, polar, non polar and wax packed mid polarity column. I took all kinds of small vials to try out, they had also done
a load of runs to test it out properly after servicing and calibrating it.
The machine was originally from SHELL the petrol people funny enough.
Dont ask me, I only know enough to be dangerous
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
How the heck did you get them to let you buy it??? Sheesh, I want one...!!!
|
|
|
Little_Ghost_again
National Hazard
   
Posts: 985
Registered: 16-9-2014
Member Is Offline
Mood: Baffled
|
|
Well there is something here my dad runs called the Glenluce Primary Project, its a science/ecology project for kids aged 6-12 (sometimes 14).
Its a kind of science learning center, three times a week there are lessons after school and at weekends families that are registered can come and
wonder around the ecology trail etc and use the center for stuff like pond dipping.
Its not complete yet and still very much a work in progress, its a not for profit thing and the kids dont get charged anything.
The idea is to give the local children better facilities for science etc.
Someone who works for shell servicing there lab equipment got in touch with my dad, he had one listed on ebay but it was decided it wasnt suitable.
Anyway this guy mentioned that Shell was getting rid of alot of there laboratory equipment in a upgrade, and some was up for grabs for good causes for
a massively reduced price.
So after alot of talking to him he got together a series one machine and vamped it upto the series 2+ with some added extras like MS and PC control,
split/splitless injector, 3 columns and two brand new bottle regulators.
Its a great system that under normal situations we would never be able to afford, we also received a large donation for the center that paid for most
of it.
For tax reasons etc its actually owned by the company that is run from here, but will see use by me and where applicable by the science center.
You can pick up a straight HP GC in the uk for around £450 but thats without a MS and the pc control system, so you would need to faff with the front
panel settings and get a paper recorder if you can find one).
We looked at many systems and in the end had we not of got this system we would have done the following.
Get a HP 89xxxx series 2 cost more but series 1 is just as good if you not going the pc route. I wouldnt use the GPID connection straight to a pc
card, I would get a control unit that sits between the GC/MS and the MS, then use a GPID to serial cable (25 pin) between system and controller. Then
you just need a serial cable from controller to pc.
The best software IMHO is total chrom from elmer perkings, it will run the HP no problem and has some great features while being easy to set up if you
dont want to get right into it.
Dont buy a elmer perkins GC or one the obscure ones, they are not worth it.
We were offered a auto sampler but after alot of reading it was decided they can be more trouble than there worth unless you have them set up
perfectly, also they destroy the septum pretty quickly.
You probably didnt want to know all this but just in case..............
But in the UK a Decent HP89xxx system should go for £450 for the basic thing, for a full GC/MS system thats pc controlled etc then your looking at
around £11,000 for the full system.
We didnt pay anywhere near that, for ours but we were lucky with SHELL.
Then you have the bottles, this has proved a pain in the arse to get.
Carrier gas is mostly a choice between Hydrogen (the best but you really need a gas detection kit with it, this shuts down the system if you get a
column leak), Helium, nearly as good as Hydrogen but pure helium at the moment is VERY expensive in the UK at the moment, a full size bottle cost you
around £520 for the first bottle then £14 a month rent, if you dont have the regulators then factor in around £180 for a decent set. Then there is
Nitrogen as a carrier, this is the option we went for, the cost of the first bottle is around £180 (full size) for pure nitrogen refills though are
only around £60 and the rental is £9 a month for the bottle. Regulator for it are the same as helium for a twin regulator.
Air can be bottle but we are going down the jenair compressor system used by dentists, a decent no non iol one you can get for around £300
secondhand, but over time works out way cheaper than using bottled air.
The MS we have isnt connected at the moment, this is because we were given a choice, have the MS and the NIST database software (around £500 for the
software normally), or just have the MS and no database software and instead they threw in 2 other different columns, we went with the columns because
they are HP ones and worth over £400 each.
The columns I chose cover most situations. The MS database isnt really an issue, I can connect it and as standards get run through the machine I can
manually add them to the empty database.
Sorry for the long post but it might help those wanting a system.
I am in touch with another guy and can get serviced and calibrated HP machines at a good price as well as HPLC systems.
He is a service engineer for this stuff and does up old non working systems.
We were also offered another system from Shell that was a Agligent, but was way above what we needed and the cost was double what we paid for the one
we got (still way lower than it should cost).
So that is how I got a GC. Apart from light use at the center I will use it alot for my soap business as soap is now classed as a cosmetic and the
regulations are tight, because I make cold process soap out of plant extractions etc, it was essential I had a GC so I could be sure I knew what was
going into the soap I make.
I have made soap since a small kid but now I am selling it instead of just entering contests, also dad uses the GC for a side business I am setting
up.
LG
I will post pics of it when I get the Gas delivered, its a really smart system.
Dont ask me, I only know enough to be dangerous
|
|
|
zed
International Hazard
    
Posts: 2277
Registered: 6-9-2008
Location: Great State of Jefferson, City of Portland
Member Is Offline
Mood: Semi-repentant Sith Lord
|
|
Gasoline composition varies by season, and also by geographical location. I have seen reports stating that Aussie gas contains a large percentage of
aromatics.
Easier to work with, might be paint thinner. At my local hardware store, in Oregon, it sells for ~10-15 dollars per gallon. I am under the
impression, that it more or less conforms to the hydrocarbon profile of what we often call "Petroleum Ether".
[Edited on 30-11-2014 by zed]
|
|
|
careysub
International Hazard
    
Posts: 1339
Registered: 4-8-2014
Location: Coastal Sage Scrub Biome
Member Is Offline
Mood: Lowest quantum state
|
|
Quote: Originally posted by zed  | Gasoline composition varies by season, and also by geographical location. I have seen reports stating that Aussie gas contains a large percentage of
aromatics.
Easier to work with, might be paint thinner. At my local hardware store, in Oregon, it sells for ~10-15 dollars per gallon. I am under the
impression, that it more or less conforms to the hydrocarbon profile of what we often call "Petroleum Ether".
[Edited on 30-11-2014 by zed] |
The whole purpose of using petroleum ether is, I think, to get a "low" boiling point hydrocarbon mixture, where "low" can be anything from 30 C up to
120 C, depending.
Paint thinner is not going to have any low boiling fractions I think. VM&P Naptha ("paint thinner" for many) seems to range from 120 to 200 C. It
also is no longer available off the shelf in Southern California, and some other parts of the U.S.
Some lighter fluids seem to be a good pet ether substitute. Zippo looks like it has a BP of 85 C, Ronsonol is higher (150 C). Pure n-Heptane (BP 98 C)
is available as "Bestine" solvent in art stores, with a pretty good price (ArtSupplyWarehouse had a gallon for $45 last I checked, about the same per
ounce as Zippo in 12 oz cans).
Gasoline refiners like to put as much low boiling stuff into gasoline as they can because they have a surplus of the stuff. This is why gas prices
drop in the winter when the "winter blend" starts shipping. They are restrained by air quality regulations, but winter blend gas should have a good
proportion of low BP hydrocarbons even in California. If you strip them from gasoline with a still of some sort, and then burn the leftover gasoline
then you are only paying $3.50 (or whatever) per gallon for the "petroleum ether".
|
|
|
Refinery
Hazard to Others
  
Posts: 371
Registered: 17-2-2014
Member Is Offline
Mood: Still
|
|
I was thinking of cheap non polar solvents that I could possibly need in a bit larger scale (5-20 liters) so any reagent is out of limits due to cost
and the unimportance of the actual process to render it unfeasible to even do.
So there is the gasoline, a bit over buck per liter straight from the pump by the gallon.
I was thinking, I could first wash it with water to remove ethanol and some water soluble stuff, and then distill everything below ~80C to get a non
polar solvent that can be used to separate stuff from aqueous phases and distilled off without too much trouble.
Should this be feasible to do? I'm not too keen to know what's in it, as long as it does the job. Damn, most of world's drug supply is extracted with
gasoline so it most definitely works and is as cheap as it can get, although those guys don't use PPE and waste mangement is served by the nearest
pond in the jungle. But you get my point?
Now.. On the very same topic. I must ask a question. Why is gasoline not fractionated and used more often as amateur chemist solvent? Like it is
stated in the topic, it can contain a significant amount of fractions between 30-80C, and apparently they do a good job at extracting many compounds
and they are insoluble to water, is freely available by the bulk very cheap?
[Edited on 12-5-2020 by Refinery]
|
|
|
Johnny Windchimes
Hazard to Self
 
Posts: 61
Registered: 28-5-2019
Member Is Offline
Mood: Sorry, it's my chimes~!
|
|
I can add that I found these videos useful, and I do love that channel for it's humour as well heh....
Distilling Gasoline
https://www.youtube.com/watch?v=FP5JaSH4ysA
Extracting Hexanes etc from Gasoline
https://www.youtube.com/watch?v=UUYu4sEwdTw
~Incredibly profound and/or wise quote goes here~
|
|
|
Refinery
Hazard to Others
  
Posts: 371
Registered: 17-2-2014
Member Is Offline
Mood: Still
|
|
I watched these indeed earlier when I considered this a viable source for hexane, but apparently the other fractions are as suitable for extractions
and only a few % of gas consists of hexane so it would be waste to extract only it if the solvent has an actual function other than curiosity.
|
|
|
draculic acid69
International Hazard
    
Posts: 1371
Registered: 2-8-2018
Member Is Offline
|
|
Petrol should be iso-octanes shouldn't it? And isn't the petrol fraction 8-16 carbons long?
|
|
|