| Pages:
1
..
18
19
20
21
22
..
48 |
tentacles
Hazard to Others
  
Posts: 191
Registered: 11-11-2007
Member Is Offline
Mood: No Mood
|
|
I should add that you can use that MMO mesh for the cathode material as well - it's perhaps a bit of a waste but there are benefits to using it as a
cathode material, and it's not like you're going to be in short supply of the stuff.
|
|
|
y2kbugger
Harmless

Posts: 16
Registered: 20-1-2009
Member Is Offline
Mood: No Mood
|
|
Yea I saw in his feedback someone told him that they were MMO, funny that it was one of you guys!
I am sure the insulating material inside the cooler Is Syrofoam, but it is some sort of plastic around it.
Nope its a switching supply, and non variable. I was thinking of wiring up a pulse controller to a couple power Mosfets.
That's an idea, I was planning on using SS knives, do you think it would be to much stress to run 30A on half of that anode, I'd planned on cutting it
in half and putting them both in a single plane all in the cell at once.
PS: what about greater spacing in the cell to raise the needed V?
[Edited on 21-1-2009 by y2kbugger]
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
Tentacles, I didn't know that was the guy you bought those from. Have you tried to make chlorate yet with them?
Edited to add: Rosco, check out the new "crock pot" thread - thanks!  That
thing was ridiculously easy to hack. It's very interesting, the temp controller I made was a bit of a PITA, but now that it's put together, it's a
great addition to the shop. Attach anything to it that draws less than about 25 amps, and it'll control beautifully. I've bought a 2 quart crock
pot, and another is 3 quarts. They are CHEAP. That
thing was ridiculously easy to hack. It's very interesting, the temp controller I made was a bit of a PITA, but now that it's put together, it's a
great addition to the shop. Attach anything to it that draws less than about 25 amps, and it'll control beautifully. I've bought a 2 quart crock
pot, and another is 3 quarts. They are CHEAP.
Sorry, I can't seem to stop editing... anyone have any recommendations for inexpensive USB voltage and temperature data loggers? There are a couple
on eBay - most of them are temp and humidity. I'd really like a 3 or 4 channel device that will do temp, current, and voltage. It'd be a great
addition to the lab, especially being able to graph and overlay data with things like Cl- ion concentration.
[Edited on 21-1-2009 by Swede]
|
|
|
tentacles
Hazard to Others
  
Posts: 191
Registered: 11-11-2007
Member Is Offline
Mood: No Mood
|
|
y2k: a 15" long piece of that sheet would want more along the lines of 200A, not 30. You might look at attempting a coaxial arrangement - basically a
stack of plates, with the outside plates having the actual electrical connections. It may or may not work with mesh.. I expect it might not, or at a
reduced efficacy.
I haven't tried that mesh material yet. Need microwave! I might just have to actually start putting some effort into that project. At the moment I've
been spending a bit of time upgrading my milling machine.. I finally got around to filling the column with concrete the other day, now I need to make
a better washer for the back (or better yet a backing plate) and the belt drive conversion. At least I have enough parts to make the conversion,
except the actual belt.
|
|
|
y2kbugger
Harmless

Posts: 16
Registered: 20-1-2009
Member Is Offline
Mood: No Mood
|
|
when you say "want" do you mean could handle, or that a lower current density would yield less efficiency?
Can these MMOs handle perchlorate production without excess wear? Also, can I bend this material I assume the oxides are brittle?
I'm not sure I understand what you mean about the electrode arrangement, when I think of coaxial, i think of concentric circles.
thank guys you are a real help with this.
~y2k
[Edited on 21-1-2009 by y2kbugger]
|
|
|
tentacles
Hazard to Others
  
Posts: 191
Registered: 11-11-2007
Member Is Offline
Mood: No Mood
|
|
That first question is a doozy. The bottom line is that you don't want too little current density, or too much. I think MMO typically shoots for
200-400ma/cm2. Swede mentioned his 2x6" anode was rated for 60A, and that is right about 400ma/cm2.
As far as we know, MMO does not make perchlorate. That doesn't mean it can't, just that we don't know for sure if it can, or what MMO material would
be required to do so. There are reportedly MMO materials that contain platinum, and we would assume they can make perc, but longevity/efficiency is
unknown.
As to an explanation of coaxial cells, I can't find it at the moment. Hell, I can't find *anything* these days - I was looking for that chick's thesis
paper last night and came up with nothing. What thread was that posted in again? Anyways, here's a vague picture, third post up from the bottom of the
page:
http://www.sciencemadness.org/talk/viewthread.php?tid=5050&a...
[Edited on 21-1-2009 by tentacles]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
MMO reportedly does indeed make perchlorate and Bismuth
is the catalyst which improves even Platinum as an anode, where platinum itself becomes a substrate for a bismuth doped MMO working coating. Even a
metallic Pt-Bi alloy
is superior to pure platinum for an anode. Sheesh what do I have to do here to peddle bismuth, when it seems to be the one thing that is reportedly
the best for catalytic activity favoring perchlorate production. Is it something about the name "bismuth" which just turns people away
Maybe we should rename it "mojo metal" and press on 
I have been looking at "conversion coating" schemes for
Titanium substrates, following up an idea I mentioned early on in this discussion along with others about the possibility
of getting titanium to react as a reducing material towards
tin or other metal salts, electrolessly plating a metal to metal
interface on the Ti substrate which could then be baked to the oxide. My idea was to mix some abrasive grit with the
precursor salt liquid to make a paste sort of like valve grinding compound, maybe with glycerine instead of water, and then to wet sand the rotating
Ti substrate with the
mixture, to "flash plate" an electroless metal interface plating
simply by double decomposition, reduction reaction of the
naked Titanium with the precursor salts. This is done for
aluminum machine parts, applying a "conversion coating"
to aluminum, which is a Tin-Cobalt-Bismuth alloy, via a
simple dipping process, which "case hardens" the aluminum
with a cold process chemically applied alloy wearing layer.
The same process is probably workable on titanium, and those are precisely the three metals of interest which are
most likely to produce a desired result on aluminum or titanium.
[Edited on 21-1-2009 by Rosco Bodine]
|
|
|
y2kbugger
Harmless

Posts: 16
Registered: 20-1-2009
Member Is Offline
Mood: No Mood
|
|
ahh, now that setup makes sense, thanks. 
cool, well i think i will start off with just chlorates using the mesh for + and -, i'll cut it into some smaller pieces.
I'll let you guys know how it ends up. Any one care for pics, or are simple cell like this of little interest?
[Edited on 21-1-2009 by y2kbugger]
|
|
|
tentacles
Hazard to Others
  
Posts: 191
Registered: 11-11-2007
Member Is Offline
Mood: No Mood
|
|
Rosco: Nothing wrong with bismuth, but so far it doesn't seem to incorporate easily into the schemes 'we' have been experimenting with (in a
non-theoretical way (: ). We'd all certainly love to have sweet Bi doped MMO anodes from hell whose biggest problem is storing the heaps of
perchlorate they make.
His question about MMO (I think) clearly referred to the commonly available types, which would be the bog-standard RuO2/TiO2 chlorinator/chlorate type
anodes. We have no substantial evidence to support their producing perchlorate. The one guy who claims to have done it is (in my opinion) very
unreliable as a source. Patents are unreliable at best. If standard MMO will make perchlorate, the wear rate will be unacceptable - likely even at the
price I paid for those sheets.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
MMO is a more generic term than just Ruthenium doped Ti.
And yeah if I recall the oxygen evolution and/or chlorine evolution voltage is too low and those coatings will not preferentially produce perchlorate.
I think it is a very few things which will do it and Bi is one of them. The problems encountered are pH related, and predictable. Bismuth
must be complexed or chelated or peptized to a sol, rather
than trying to use its commonly soluble simple salts which are too easily hydrolyzed to insoluble forms except in
extreme pH conditions, which are not kind to a Ti substrate. An assortment of soluble bismuth preparations
are published, so it is just a matter of choosing one which has a chance of working at the pH of your coating composition or electrolyte. And yeah
you have to go to extra trouble to complex the bismuth precursor, but only if you intend for it to work. If not then you just dump some
of nitrate into your mix and watch it precipitate an insoluble hydrolysis product ...and then pass unfounded judgement upon it as not being workable,
before you have even tried what might work instead. You can't go against the physical chemistry which applies to the precursors which are unforgiving
about the window conditions required. To make a workable MMO perchlorate anode will very likely require some attention to the Bi doping precursor,
and where there is a will there is about a dozen different ways of making it work.
[Edited on 22-1-2009 by Rosco Bodine]
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello folks,
Used a deep fat fryer for some time as a heater for round bottomed flasks. Had it up to near 200C with 'liquid paraffin' (veternary product) with
woeful smoke. 'Controll' was with a variac with internal thermostat on DFF disabled. Are Crock pots similar to slow cookers?
The co-axial arrangement that is being mentioned above is not a co-axial arrangement but a series of flat plates in parallel with just electrical
connections going to both end plates of the stack. Each plate is an anode on one side and a cathode on the other side, except the end plates. It's
called a bipolar cell (as opposed to monopolar where each anode and cathode has an actual current carrying wire going to it). It's OK to use MMO as
both an Anode and a Cathode as it is done in the bipolar cells, expensive though.
I think we should reserve the Mixed Metal Oxide (MMO) term for anodes using Noble metal Oxides on a valve metal, (Ru and Ir with perhaps Pt, Oxides
making up the vast majority) otherwise LD or Magnetite etc etc with just about any metal Oxide will be an 'MMO Anode'.
@y2kbugger
You can bend all you like. It the MMO chips off it will be no big deal as the Ti will protect protect itself with it's own Oxide, that's why it's
called a Valve metal.
We love pics too!
Has anyone here actually established that the 'Ti' mesh sheets on sale (the ones about a meter long and bent at right angles all the way along)
ACTUALLY HAVE an MMO on them???????????????????????? Perhaps I missed the post?
Perhaps they do not have MMO on them at all  . .
Sedit said this:
____________________________
I cant say that i have read through this whole threed but i do know for perclorite production that this threed started about I have read about alot of
gold electrodes being used
____________________________
Where did you read that?
Where there's a will............................................................
There are relatives :-|
Dann2
.
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
When it comes to making bismuth soluble, there are the polyol complexes with glycerin, mannitol, sorbitol, and erythritol, and there is also glycine,
nitriloacetic acid,
EDTA, and of course citric acid, acetic acid, and ammonium nitrate....and the list is growing.
|
|
|
y2kbugger
Harmless

Posts: 16
Registered: 20-1-2009
Member Is Offline
Mood: No Mood
|
|
i got mine yesterday, and i an 97% sure its mmo, because its flat black, and in the above post some else who bought from the guy also confirmed it.
noone has actually run one yet tho. or have they?...
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by y2kbugger
i got mine yesterday, and i an 97% sure its mmo, because its flat black, and in the above post some else who bought from the guy also confirmed it.
noone has actually run one yet tho. or have they?... |
Someone needs to... cut a small piece, and run it furiously down to low chloride ion concentrations to see how sturdy it is. The next step is running
it in saturated chlorate... not to see if it makes perc (it probably won't) but just to see if it holds together well.
Apparently the guy has a lot of it, no doubt as surplus from pool chlorination.
Does it look new?
|
|
|
tentacles
Hazard to Others
  
Posts: 191
Registered: 11-11-2007
Member Is Offline
Mood: No Mood
|
|
My sheets appear to be new, aside from a bit of shipping damage, a few small places where the coating rubbed off as the sheets abraded each other. The
appearance is identical to the MMO that Swede sent me, but that doesn't tell the story of what's in it, what sort of formulation it is.
|
|
|
y2kbugger
Harmless

Posts: 16
Registered: 20-1-2009
Member Is Offline
Mood: No Mood
|
|
I found mine to be in that same condition, a little scratched here and there.
well I decided to try to purify my graphite run, I mixed in some kcl solution, and got it to ppt about an inch or 2 of suspended crystal in a mason
jar. My question is how do yo guys filter stuff, i used coffee filter but it seems the basic solution eats at the paper alot, or just the fact of it
getting wet maybe, whatever the cause, it really tends to rip. i estimate the crystals that are drying now will be about 100 g but i could be way off.
thats from a 2 week 1 amp run.
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
y2k, I hate plugging myself but I've made a lot of potassium chlorate from MMO and blogged it all. If you go back and forth in time from here:
http://www.apcforum.net/forums/blog/swede/index.php?view=sho...
you'll see one way that it can be done. I use KCl as a starting point, and what that does for you is grow really fat crystals, easily filtered, as
opposed to a decomp of sodium with potassium, which I suspect produces powdery precipitate. My best "filter" is a 4 liter food container with about a
thousand small holes drilled in the bottom. All the goodies go in there, and the crystalline mash is washed with ice water and alcohol. Tests showed
that the washing does a fine job in removing excess chloride and other species... the potassium chlorate ended up being 99.3% pure, as best I could
tell.
Most guys will advocate using sodium chloride as a feedstock, proceeding to sodium chlorate, then crystallizing and purifying the sodium chlorate to
act as feedstock for a perchlorate cell. The solubilities are vastly superior to the potassium variety. OR, they attempt a one-shot, straight from
chloride to perchlorate process which I personally don't care for, as my feeling is, it is very hard on anodes, and we have enough trouble with anodes
as it is. The less chloride a perchlorate anode is exposed to, the better.
So far I have had very good luck using only potassium. I make up for the low solubility of the salts with cell volume. Since it makes crystals
during the run, any fancy circulation setups can be problematic, as they do jam strongly with potassium chlorate crystals.
There is no substitute for raw amps. For chlorate, I had zero problems doing 60 amps on a 6" X 3" MMO anode, but a heat sink on the Ti strap was
required, or the Ti strap would heat to the point where it would melt any plastic it contacts. 60 amps in a big cell makes a kilogram a day.
The MMO I have does NOT make perc; or, if it does, the efficiency is about 2%. I strongly suspect the stuff you guys got is standard pool chlorinator
MMO, which will work great for chlorate, but will not make perc. Several days of high amperage through a ruthenium-based MMO produced chemically
detectible traces of perc, whereas a Pt anode (same conditions) by then had dropped two inches of potassium perchlorate ppt to the bottom of the cell.
The other, odd difference between the two - the Pt cell created an ozone smell, whereas the MMO perc cell, NO ozone smell.
I have not tried the lead dioxide anode yet.
Roscoe, I have on hand the following: TWO different bismuth salts, CP titanium tube, EDTA, citric acid, pretty much everything you mentioned. Tell me
what to do (spoon feed me) and I'll try making a Bi anode. I also still have the three kilos of boehmite, singing their siren song, "use me! uuuuse
me!" But I wouldn't know where to begin.
I added a monstrous stirrer to my plating rig. It ended up looking nice. The stainless adapter on the motor will accept any 3/8" shaft, or I can
turn down a shaft to 3/8" to adapt. One option is 3/8" Ti... I did turn down a 3/4" shaft of PET plastic for it, and all I have to do now is slot
the end and install a paddle with a Ti screw.
Of all the plastics I've tried, PET is my new favorite, it's awesome stuff! Cheap too. I'll reach for PTFE ONLY if the chemical resistance is
needed, but PET is very good in that department.
Observation: anything metallic in the bath other than the anode ended up growing Cu fuzz or plate. My cartridge heater sealed in a Cu tube had a
rather heavy Cu plate on it. Stray voltages? Do I need to ground the bath with a Ti probe tied to earth ground, or simply ground the immersion
heater? Or is the Cu depositing non-electrolytically?
Interestingly, the supposedly unplateable Ti cathode had a pretty firm Cu plate, but a bit of hand work and it peeled off pretty easily.
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
Some microphotographs of anode 2. Under a Meiji scope, the surface looks interesting, and promising. These pics are nothing more than a Sony camera
held up to the eyepiece, set on manual, with a bit of fiddling on exposure and f-stop. The coating is BLACK, but can look to be different colors in
these pics.


Zooming in revealed a crystalline structure that looked like velvet, for lack of a better term:


And as I mentioned earlier, I decided a vigorous stirrer would be best for a plating bath. The motor is 24VDC, gobs of torque, yet turns over at 3
VDC, and can be varied infinitely in speed and power:


I used a lot of loctite both for the vibration mechanism, and this stirrer, to keep everything secure. The vibrator motor can easily back a nut off
if care is not taken.
All I need to do now is come up with a compact paddle setup that will gently stir, yet not toss toxic electrolyte everywhere.
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
LONG LIVE THE POOR MAN'S MMO
Howdy folks,
| Quote: | Originally posted by Swede
Most guys will advocate using sodium chloride as a feedstock, proceeding to sodium chlorate, then crystallizing and purifying the sodium chlorate to
act as feedstock for a perchlorate cell. The solubilities are vastly superior to the potassium variety.
OR, they attempt a one-shot, straight from chloride to perchlorate process which I personally don't care for, as my feeling is, it is very hard on
anodes, and we have enough trouble with anodes as it is. The less chloride a perchlorate anode is exposed to, the better.
So far I have had very good luck using only potassium.
|
Coming from a direction where you only have Graphite for making Chlorate then the Sodium salt is the way to go as it is difficult to get the Carbon
powder (black shit) out of the less soluble K salts. All that changes with squeeky clean MMO.
The one shot all the way to Perchlorate may be a bit of a pipe dream. Industry got 64% CE (using pH control). Accounts in the Journals I have read
give 50% (using pH control). We will get half that (and less in my experience) using no pH control with wear on LD anode. Perhaps with pH control it
will be much better. More CE guraranteed and hopefully less erosion of LD.
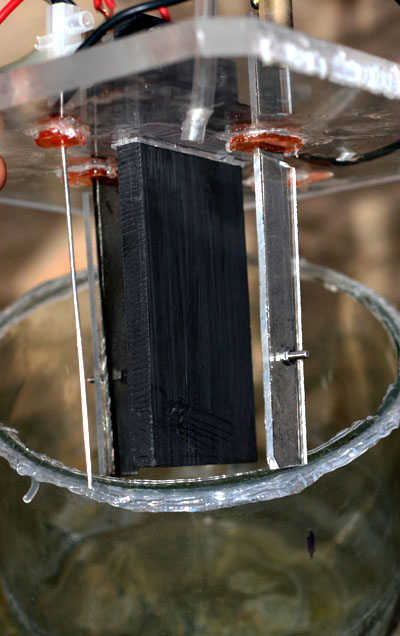
Going off on a bit of a tangent, I am about to set up a pH controlled cell using <FONT> <UNDERLINE>POOR MAN'S MMO</FONT>. Control of
pH will consist of simply adding 12% HCl at 1.2ml per hour into the cell. 4.5 amps, thats 30 something mA per square cm on anode.
Simple glass jar cell, computer PSU, perspex lid
Will be using four small cathodes with the backs of the flat cathodes covered with plastic. This keeps current density high on cathodes and will
reduce hypochlorite reduction. Cathode are Ti so there should be no reduction of Chlorate. (as stated elsewhere in some thread somewhere).
I will be using no additives like Chromate, F etc, ie. a green cell!!
Hopefully there will be little Graphite (POOR MAN'S MMO) erosion. It has been reported as low as 4kg per ton Na Chlorate (4 grams per kilo  ). That would give MMO a run for its money eh. ). That would give MMO a run for its money eh.
Forgot to ask:
Swede, can you see any pin holes in the LD when looking at it under the microscope? I was surprised to see many on the anode I made. They were not
visable to the naked eye.
Dann2
[Edited on 24-1-2009 by dann2]
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
@ Swede, what bismuth compounds have you got ?
About pH control for perchlorate, reportedly it is not necessary for a cell having a nickel cathode and a PbO2 anode, after the point where the NaCl
is all converted to chlorate.
See attached patent US2840519
Attachment: US2840519 optimized Perchlorate by PbO2 and Ni cathode.pdf (171kB)
This file has been downloaded 734 times
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
@Dann2: I like your setup! Healthy slab of graphite plus high current density Ti cathodes. That is one thing I was unaware of when I was
constructing my cells, the need to keep cathode density high, thus I made giant Ti cathodes that were a waste of material. It still worked, but the
efficiency was probably reduced as a result. It looks like 2 of the cathodes are Ti wires. An alternate setup would be about 8 of those wires in a
pattern around the anode. Or how about "U" shaped Ti wire cathodes? Install from the bottom, with each "U" of course requiring two holes in the
perspex. Where all the "U"'s intersect at the very bottom, below the anode, wrap a few turns of Ti wire to secure. Tie them all electrically
together on the dry side, and there's your all-wire cathode cage. Such a cage would work well for LD plating, too.
"Green cell" I like that too! I've never added anything to my cells yet, either, and have no problem sacrificing efficiency for safety. Chromium
and NaF... yuck.
I understand the need for sodium when using graphite, but am I to understand you are attempting potassium here?
HCl: With "T-Cell Jr" (18 to 20 liters) I had the dosing timer set up to turn on 6 times per day for one minute, and each cycle of the dosing pump
delivered 12-15 ml, so somewhere around 100 ml per day of 15% acid worked. I could probably have added more, the pH was more often than not around
7.5 rather than 6.8, but I was very pleased at the stability. Once it was "forced" down to near neutral, there was no tendency to rapidly climb;
periodic acid dosing as a concept works, and I believe it is a good alternative to full pH control with an immersed probe, with its associated probe
poisoning problems.
No pinholes on the LD!  Between rotation + vibration + maybe surfactant, I
believe it created an environment in which bubbles simply couldn't stick. I'm hopeful strong stirring will also help create a quality plate. Between rotation + vibration + maybe surfactant, I
believe it created an environment in which bubbles simply couldn't stick. I'm hopeful strong stirring will also help create a quality plate.
RB, For Bismuth salts, I have maybe 1 lb each of Subnitrate and Hydroxide.
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
@Swede.
The cell I have set up is an Na cell just to see what erosion I will get with pH control. When conc. of Chloride gets low (100 grams per liter approx)
I will change the cell contents and run again perhaps twice more. Testing anode more than actually making Chlorate.
I'll do a K cell then.
This cell has been going now for about 9 hours and soluton is crystal clear.
Often wonder about the Manganese anode (Xenoid ®) in a pH controlled cell. It might last 10 to 20 times longer.
After that I will recommission! the old battered LD on Ti anode and see how it goes in a pH controlled set up. It has 3 months clocked up in
uncontrolled cells and has been sitting on the bench for the last 4? months. If if still goes OK, I think it is fair to say it is a very good anode
making method for the Garage Guru.
I have been told that LD has low catalytic activity so it may perform much better in a pH controlled cell (no Anodic Chlorate making required, just
Chlorine evolution).
Thanks for the acid info. I was looking for it actually.
You seem to have been able to control cell with a very small amount of acid compared to my (much smaller) 5 amp, 2 liter cells.
Your cell needed 0.104ml 12% HCl per hour per amp to keep pH about 7.5.
My cell needs 0.42 ml 12% HCl per hour per amp to keep pH at 6.8.
About 0.39 ml per hour per amp keeps pH at approx. 7.5.
(I converted your figure to 12% acid as that is what I have, from the hardware store).
Cheers,
Dann2
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by dann2
Thanks for the acid info. I was looking for it actually.
You seem to have been able to control cell with a very small amount of acid compared to my (much smaller) 5 amp, 2 liter cells.
Your cell needed 0.104ml 12% HCl per hour per amp to keep pH about 7.5.
My cell needs 0.42 ml 12% HCl per hour per amp to keep pH at 6.8.
About 0.39 ml per hour per amp keeps pH at approx. 7.5.
(I converted your figure to 12% acid as that is what I have, from the hardware store).
|
Some variables at play here... sodium vs potassium salts? Shouldn't cause much of a difference, but the mysteries that occur at the anode in a K vs.
Na cell, chemically, may account for a bit of that. And as you noted, my pH was still a bit high. I cut the 30% pool acid in half... for the next
run, I'll leave the timer alone and use 20% acid rather than 15%, and I suspect the pH control will be better.
In a small cell, I think you may have to watch out for less pH stability... it'll have a tendency to climb, faster, than a larger cell. Tentacles and
I were discussing a drip setup, like a hospital IV bag, with the drip slowed down dramatically so as to deliver the correct dosage throughout the run.
Or, one could use a solenoid valve in a gravity setup, using a fine-bore tube, with the solenoid set on a timer. One last thought is a "diffuser
tube" which I had in fact installed on my big cell. It's nothing more than a vertical section of PVC tube, sealed at the bottom, cross-drilled with
very fine holes, and the open top is above the surface of the liquor. The added acid goes into the tube at the top. and the acid diffuses very slowly
into the system through the tiny holes. All of those help avoid a pH roller-coaster by delivering small amounts of acid nearly continuously.
Finally, there's that patent that discusses mono and di-sodium phosphate to buffer the system and make pH MUCH easier to control. That I think is
more of a player with K feedstock, as the buffers would then get washed away during crystal processing... although much the same would happen when you
add KCL to convert and ppt the k-chlorate.
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello Swede,
Chromium also buffers the cell. I am not using any.
From the pH controlled Graphite anode cell I have good news and weird news.
First the weird.
I was adding acid at quite a large rate, as stated above, and increasing the rate so to get pH down to 6.8. The rate had gone up to 0.42ml per hour
per amp. The pH dropped to 6.7 so I decided to turn off the acid to let pH increase a bit. I assumed that when acid was turned off for about an hour
or so the pH would go back up.
24 hours later with no acid going into cell the pH had increased from 6.7 to 6.8!!
The cell seems to be staying rock steady at the wanted pH without adding any acid.
WTF,,,,,,,, beats me. Anyhow I turned the acid back on at a very low amount (about .05ml per amp per hour) after the 24 hours.
I am adding acid on a continous basis using a Grasby Syringe pump. Its a bit of an overkilll machine but great for small experimental cells.
The good news is that after two days of operation the cell liquid is CRYSTAL CLEAR. Not a single grain or speck of Graphite to be seen. It's just like
an MMO cell.
The temperature of the cell is low at 23C so conditions are not optimum for 'Chemical Chlorate formation'. I guess I could wrap some insulation around
it but am not going to bother. Will just let it run its course (approx 5 days).
Thats another advantage with using MMO when controlling cell pH in a one cell system. You can let the temperatrue go way up to 70C or so and since MMO
is so tough it does not seem to get damaged. 70C would probably erode Graphite too much.
To get the best from Graphite you would really need a two compartment system. One compartment with anode (below 35C or so) and another larger
compartment with a higher temperature (70 - 80C) for to achieve optimum conditions for the 'Chemical Chlorate formation' to take place.
I think the lesson to be learned from this cell is that if you want to avoid the mess with Graphite and have the anode last long and long then
controll the pH. iT's early days yet and the anode may start to erode after some time.
Dann2
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
@dann
Sedit said this:
____________________________
I cant say that i have read through this whole threed but i do know for perclorite production that this threed started about I have read about alot of
gold electrodes being used
____________________________
Where did you read that?
I thought I read it in a ECS paper but i must have been mistaken because attempts to find it again turned up nothing and I found this web page that
states the compleate opposite that gold cant be used at all for perchlorite production.
Never the lest thought this is not a bad web page that states the pros and cons of chlorite electrodes.
http://www.geocities.com/CapeCanaveral/Campus/5361/chlorate/...
Hell they even have a section of Diamond electrodes which I havent even heard of.
|
|
|
| Pages:
1
..
18
19
20
21
22
..
48 |