| Pages:
1
..
22
23
24
25
26
..
48 |
Bikemaster
Hazard to Others
  
Posts: 120
Registered: 8-10-2008
Member Is Offline
Mood: No Mood
|
|
Maby the perchlorate was not made by the anode???
If the crystale shape don't really mather, I can suppose that the result of my cell is only kclo3 (+some inpurity).
So the problem can come from the waching phase and the drying phase.
1. filtration: I only filtrate the solution and i roughtly washe the crystal with cold water.(don't make any recrystalisation) (can have some kcl
inpurity)
2. drying : I let dry the the filtrate in a oven wicht run at 140C, and i am not sure but can some kclo3 turn to kclo4 at this temperature (can be at
this temp for over 6 hour). If yes it made some kclo4 and kcl... after drying i never wash a other time so i can have like 10% of kcl of impurity in
total witch is not very good.
before to have mmo (cell with graphite anode), the kclo3 was dry under a light (less heat but longer) and it is when i make this switch that i saw the
difference...
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
Bikemaster, I've thought of another way you can get an idea if you have potassium perchlorate, or chlorate - check the solubility. It won't be exact,
but it might give you a rough idea. Potassium perchlorate is soluble at 100 degrees at the rate of 218 grams per liter, while the chlorate is soluble
at 570 grams per liter. You could possibly kill two birds with one stone - recrystallize to get rid of the chloride, and see how much water it takes
to dissolve your crystals.
I'd be very surprised if even a tiny portion turned into perchlorate at 140 degrees.
|
|
|
Bikemaster
Hazard to Others
  
Posts: 120
Registered: 8-10-2008
Member Is Offline
Mood: No Mood
|
|
Ok I just recrystallize roughtly 100g of my last batche.
First, the recrystallize crystal have no more the shape of the precipate in the cell, the crystal have now the square shape, wicth is kclo3 . .
Secondly, I collect the water of the recrystallization and i boiled down all the water, I collect the salt and i dry it. I mix it with sugar and made
the same mix with other of my kclo3 wicth i don't have touch. Result, the mix with the collect salt burn twice slowly that the other mix. So i can say
that it have a lot of kcl in it... the inpurity is only kcl and maby 1% are 2% of kclo4 but it dont really change the result of the reaction. i think
that i just need to wash the crystal better and it will be ok.
I think that i find the reason why it seem more stable... I feel very stupid but the reason it the shape of the flore... i test the stability of my
sample by hitting on it with an hammer. but i don't think about i thing, the type of the flore... before i was testing on rought flore and now i test
on smooth flore, wicth is more hard to make detonate.
i find that i had max 3% of kcl in my kclo3 i will only wash them more next
time and i think that it will be ok. i will only wash them more next
time and i think that it will be ok.
thank for helping me
|
|
|
tentacles
Hazard to Others
  
Posts: 191
Registered: 11-11-2007
Member Is Offline
Mood: No Mood
|
|
Are you ONLY washing the crystals from the cell, or are you recrystallizing it? I would suggest recrystallizing it at least once if you're going to
use the chlorate for any kind of pyrotechnic purpose.
|
|
|
pyro6314
Harmless

Posts: 16
Registered: 30-11-2007
Member Is Offline
Mood: No Mood
|
|
With my current running in my cell so low, only 3.0A @6.0V limited, I decided to put the electrodes closer to try and decrease the
resistance....absolutely no difference. I would hazard to guess if I turn up the voltage any more the anode is going to melt off in a big hurry. It
already is so I switched polarity. Lol
While I was at it I attempted to filter the graphite out of the glossy pitch black solution (looks like paint) in attempt to reduce erosion...Not
going to happen. It clogged my filter paper and majority went through anyways. I did dry out my filter paper and light it  There must be an appreciable amount of Chlorate in there already. Topped it up and
fired it back up. There must be an appreciable amount of Chlorate in there already. Topped it up and
fired it back up.
I don't have the patience for this icky black mess and I would like the satisfaction of crystal precipitation. My next run will be with KCl and my MMO
in the same cell. I will leave dann2 to venture into the black sludge mysteries.
@Swede (or anyone), do you have a suggestion for how big of piece of MMO and how to attach it without access to a spot welder or titanium. Doing this
sort of thing without facilities or resources is painful. Maybe I could spot weld with booster cables and my supply @ 90A 
Off topic, I'm curious how fast I can vaporize a large chunk of iron into its oxide electrolytically...
|
|
|
Bikemaster
Hazard to Others
  
Posts: 120
Registered: 8-10-2008
Member Is Offline
Mood: No Mood
|
|
Yes, i think that i need at least one recrystallisation. I go over this step because my cell seem clear of impurity but i don't think about kcl...
anyway, make one recrystalisation is not very long and it goning to make much better quality kclo3
For you Pyro, i you want to weld something to your mmo anode, you have to think to recoat it, because the spot of titanium will corode it they are in
contact with the electrolite. If you want an easy way to hold your anode (if you have mesh anode), use a titanium wire and past it in some of the top
hole of your anode. If the titanium wire don't touch to the electrolite, you will never have problem with it.
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
To spot-weld the eBay MMO requires scraping of the MMO off of the contact points. It is the only MMO I've encountered that requires that. The brand
new sheet I bought a while back does not require scraping; I simply spot weld and the weld is a good one. The spot welding points are the junctions
in the mesh... trying to spot-weld the MMO wire between junctions results in a melted wire, as it does not have the mass.
I think a good size for a moderate home cell is 2.5" X 5", or 60mm X 120mm. If your power supply is only capable of a few amps, you'd want to go much
smaller. Yes, there is a theoretical current density on the anode for chlorate, but honestly I haven't noticed any real issues if I am not close to
theoretical.
For the eBay MMO, if you don't have a spot-welder, I'd simply cut it so that it has it's own "strap" and secure the cable through the mesh using a
crimped connector, and a SS bolt with two washers, drilling the mesh if necessary so the bolt fits through. You lose a bit of MMO area, but the stuff
is cheap enough so that it's no big deal.
I had a PM from a nice guy in Australia who had his eBay MMO cut with a cutoff disk, and he said they now have a POTENT smell that gave him a
headache. I found this to be really odd. Any theories? I never noticed anything even when spot welding the material to Ti hangers, but I normally
wear a respirator when doing so.
I'm thinking this stuff (the eBay MMO) was USED in some chlor-alkali process. The 90 degree "foot" portion is a slightly different color, and I am
thinking the foot was clamped in some sort of valve-metal mounting, and not subject to any electrochemical processes. Overall, these sheets have a
brown tint compared to new commercial mesh, which is flat black.
That doesn't change the fact that it makes chlorate like crazy, but perhaps there are some foreign chemicals absorbed or trapped in the coating, or
they weren't cleaned thoroughly after being removed from service.
|
|
|
pyro6314
Harmless

Posts: 16
Registered: 30-11-2007
Member Is Offline
Mood: No Mood
|
|
The pieces I have appear to have been cut with a zip disk or grinder as well. Where the heat would have been concentrated, there appears to be an
"alkali" looking white oxidation. Inside the white stuff is silvery stuff with some kind of crystal structure. Man I wish I had Swede's microscope. I
emailed the Ebay seller and he says he has 25-35 lbs left and can get more anytime. Such an odd thing to have unlimited supplies of  I also asked what its previous application was and what the composition of the MMO is
and Im awaiting a reply. I will let you guys know. I also asked what its previous application was and what the composition of the MMO is
and Im awaiting a reply. I will let you guys know.
As for the "POTENT" vapors... kinda hard to say. Some kind of a crazy replacement reaction with the grinding wheel compound or airborne Ti oxides and
MMO. Couldn't tell you. Maybe someone will come up with something after we actually know what its composed of.
For making up an anode, I suppose I could cram a big one into my little cell then just run at a lower current density. No harm in that, correct? When
I up size then I can use it there as well. Im waiting for the guy to email me back with me Hanna Dosing pump/ pH controller. Awesome deal if I can get
it seeing as the they are in the ballpark of $850 USD retail. (Factor the terrible CAN dollar as well)
Edit: @ Would it be a bad idea to increase the voltage past 6V to get more current through with a graphite cell?
Edit2: He doesn't have any info on the mystery MMO.
[Edited on 13-3-2009 by pyro6314]
[Edited on 13-3-2009 by pyro6314]
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
I bought one of those Hanna dosing pumps, and I think it is a good deal, if it's from the same eBay supplier. The head of the pump is all PTFE, PVDF
(even the spring) and glass, and HCl doesn't touch it. The only trick is to set up some sort of a timer. The Hanna pumps come in different volumes
per stroke, and I ended up with one that delivers exactly 1ml per stroke. On the slowest speed, it does about 16 strokes per minute, so a 1 minute
"ON" from a digital timer delivers 16 ml of HCl. If you can, get the smallest one, unless you have a monster cell; or, if you have a timer that can
do less than 1 minute intervals.
I don't think you'd have any problems with a larger anode in a smaller cell. You may lose a little efficiency, but it won't be gross. But given the
size of those eBay sheets, why not make a couple of "junior" anodes? I made several, and they are handy for test runs, or if you want to try
something like taking the cell to near 0 chloride, or something else that might be harmful to the anode. You're risking less in that case.
One thing to consider - does the pump have a manual override? In other words, does it require a constantly immersed pH probe to function? We've
hashed that out I think in some detail, and I don't think a typical probe would survive long in a cell... it would become poisoned, and even a cheap
pH probe is pricey. So being able to use the dosing pump as a simple on/off device would be nice, as well as use it as a controller if you want.
[Edited on 13-3-2009 by Swede]
|
|
|
pyro6314
Harmless

Posts: 16
Registered: 30-11-2007
Member Is Offline
Mood: No Mood
|
|
Actually the one I'm looking at is a little different from the one you got. It isn't listed on Ebay right now I just emailed the guy. This one doesn't
have a timer, just the pH controller. It does have a manual override so I will just make up a PIC timer to run it at certain intervals. I believe they
all do 1mL per stroke its just the timer that varies the amount of strokes. You could probably open your pump up and build a microcontroller timer to
eliminate your intermatic/pump timer combo. Even reuse the pot already on there.
Link to the literature-> http://www.hannainst.com/usa/prods2.cfm?id=018002
It has some other nice features that can be used. Analog output for the pH reading, Aux contact that comes on when dosing (mixing?), optional pt100
for temp compensation of pH, level control so cell doesn't over flow, Acid or base control. Appears it can do alot. Hopefully the guy emails me back
though, Im not too confident he will.
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello Folks,
The latest NaCl cell with pH control, Graphite anode at (approx.) 55°C finished.
The overall CE was 79%. The end Chloride concentration was 115grams per liter of solution.
Wear on the anode was not too bad. It made the water black but the black settled to the bottom
of the container where the more of the liqued was decanted off. The liquid was clear but has the
yellow colour that seems to always be present with the Graphite anode cells. I had to filter approx.
200ml of black liquiud through the paper filter shown. The Graphite was dried and give a figure of
6.2 grams Graphite per KG Na Chlorate made. This may not be the whole story as some of the
Anode may have gone off as CO2?. I did not weigh the Anode at start (unfortunately).
A total of 200ml of 12% HCl acid was added to the cell.
There does not seem to be a huge advantage in running the cell warm as I did not get a
great increase in CE compared to the cell I ran at 20°C. It came in at 71% CE and
it was let run untill the Perchlorate point. (low CE at end of 19%).
The 'bucket' type cell design (ie. no design), I guess, has a lot to be disired when you
are controlling pH and have a warm cell.
I have cranked up my old Lead Dioxide Anode (Ti substrate) in a pH controlled cell. It
is going OK for approx. two days. There is alot of the Ti exposed. Funny thing is the Ti
is gassing. The Tin Oxide interface coat must still be there.
This may have been the cause of my low CE in my last (pure) Perchlorate cell using
this Anode. With a large amount of Tin Oxide exposed that would definitely effect CE
as Tin Oxide is a poor Perk. maker. I tried it way back. It just sits there making Oxygen. <br>
Have graph of cell run will post.
Dann2
[Edited on 14-3-2009 by dann2]

|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
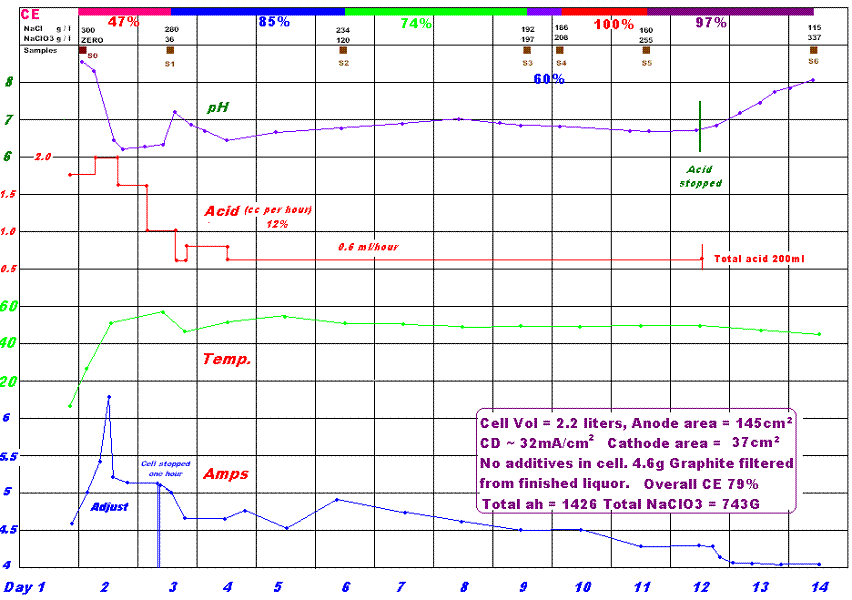
Hello Folks,
See graph below for my second pH controlled Na Chlorate cell using Graphite Anode.
The CE's are weird. I have gotton 100% CE between the last two samples. I have rechecked everything. Times, amps maths. I estimated the amounts of
Chlorate in the cel by measuring the amount of Chloride and calculating the amount of Chlorate formed from the fact that the starting concentration of
Chloride was 300 grams per liter. I did not believe the titrations/CE results so I titrated the samples again for Chlorate this time. Same result so I
am standing behind the (rather crazy looking) figures.
I am sorry now that I did not let the cell go on for longer. I though the Chloride was getting low so I pulled the plug.
I need to get a constant current supply. Varying amps is a pitb.
The acid additions you see in the graph is what went into cell. There was no 'extra' additions like my first cell run.
12% HCl btw.
The Cathode area is small with the backs of the Cathodes covered with plastic. (see picture above somewhere).
I came up with a titration for Chloride. It is (IMHO) the best poor man's Chloride titration (PMCT) ever devised. 2 grams Silver Nitrate will do at
least 80 titrations. On average the two grams wil probably do 160 titrations. Will not beat Chloride strips on handyness though. See Chloride Titration for info.
See a whopper of a supply on ebay here.
It's in the UK. Not constant current unfortunately but has a variable pot for varying the 3.3 volts out. How much I don't know. Plenty of amps!
Dann2
[Edited on 15-3-2009 by dann2]
|
|
|
Bikemaster
Hazard to Others
  
Posts: 120
Registered: 8-10-2008
Member Is Offline
Mood: No Mood
|
|
400 amp
anode will never resiste to this, no? if I compare with my anode, i need to put 8 of them in parralele if i dont want to destroye them (50 amp max).
But for this price, it is a very low price for that much amp.
I have the project to make a big cell (18 L and with 100 amp) and i was thinking about having a ph controle on this cell. If you can give my some
idea, tip and think to buy to make a ph controle.
ps. if i can save the most money, it will be fun.
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello Bikemaster,
You do not have to use all of the 400 amps of course. One problem is that the output voltage is a bit low. You would need good heavy cables and great
connections so as to minimize all voltage drops. At those currents you are going to have to have heavy cables and good connections anyways!
Two of these in series will give 6.6 volts (+ more with adjustment) which will be lots of Voltage.
Are you going to use MMO Anodes?
Regarding pH control of a cell with 100 amps going into it. From my limited experience of adding acid to cells it takes (forgetting about the start of
the cell where you need more acid) approx. 0.134 ml per hour per amp to keeep pH around the 6.8 mark (12%) HCl). At 100 amps thats 13.4cc per hour. If
you set up a tube, bottle and a diabetic syringe (with the usual very fine needle) you should be able to drip acid into your system and eliminate the
need for a pump. One drop coming from a diabetic syringe is in the region of 0.015ml (I think). That works out at you needing 900 drops per hour, 14.9
drops per minute. Could this be done with a syringe + container + tube?. You can make the rate increase/decrease by putting the acid reservior
higher/lower in relation to the needle. You could also dilute the acid more if the drop rate is too high and you cannot get it any lower. You could
perhaps pince the needle a wee bit to make it narrower to slow down drop rate.
Does anyone know how a hospital 'drip' works. You can adjust those. How are they adjusted?
Cheers,
Dann2
|
|
|
Bikemaster
Hazard to Others
  
Posts: 120
Registered: 8-10-2008
Member Is Offline
Mood: No Mood
|
|
wow thanks, this setting will cost me pready much nothing . .
I will use a 2L bottle as a container (100 amp*0.134ml*24h*6day = 1900ml) i have some 1/4 tubing wich can resist to HCL and will finish with and old
broken tip pipette (i will reduce the end hole by heating to get the right amount droping per hour (13,4ml).
some question
1.Do the water have the same consistenci that 12% HCl (will be more easy to make my test)?
2.Where do i have to drop my HCl in to the cell? Do they have better places?
3. How can you reduce the amp?with a dimer. But if it is possible, it will be a very good power supply (but better to get 2 because 3.3v it is not a
lot)
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
@Dann2: Beautiful data, well presented and a great analysis. Thanks!
Bikemaster, I think you are confused about current. A simple power supply has two ratings, voltage, and current. Most supplies are fixed voltage. A
typical one is 5 VDC. So lets say you have a 5V, 1,000 amp supply, a monster. You hook that up to a toy robot that requires 5V. The supply will not
"force" 1,000 amps through the robot. The Robot will function perfectly, and the current will be maybe 1/2 an amp. The 1,000 amp is a RATING,
meaning "This supply is capable of delivering 1,000 amps, but only if the connected apparatus will draw it."
Enter ohm's law. If the apparatus has a resistance (all of them do) the RESISTANCE will determine how much current it will draw, according to the
following formula:
Current = Voltage divided by Resistance in Ohms, or
I = V/R where I = current. If I hooked a 10 kilohm (10,000 ohm) resistor to the 1,000 amp supply, the current it will draw will be
I = 5 / 10,000 or 0.0005 amps. Not much.
You can manipulate ohms law easily to solve for any of the variables.
A CC (Constant Current) supply is a different beast. Let's say you have a CC supply rated 10V, 100A. You set it up for CC rather than CV, and start
your cell. You then dial up 10 amps; the supply calculates the attached load, and determines that 3.6V is what you need, so it delivers 3.6V.
Dialing 20 amps, you'd see something like 4.8V. Keep increasing the amperage, and at some point it won't go any higher because you'd be pegged at
10V, the highest voltage it can deliver. If you set 20 amps for a run, you'll see the voltage varying with time as the chemistry of the cell changes.
CC supplies are definitely very handy for this process, as you can control heating, and determine CE with ease.
On your cell: If the anode surface is approximately 60mm X 120mm, the cathode the same, and the spacing about 20mm, at 5V you will see that the
system will draw maybe 25 to 50 amps, somewhere in that region, depending upon the electrolyte concentration and other factors. For a 50 amp cell,
you need HEAVY cables to the electrodes, at least #8, better #6 or #4 copper, or the cables themselves will begin to heat. light cables = bad news;
overheating, voltage loss, etc. And the connection to the electrodes needs to be heavy. An alligator clip is a poor choice. A better choice is
something like this:

Where I have crimped (you can solder) a heavy Cu lug and bolted it directly to the anode shanks. Look in the electrical section of a big hardware
store for stuff like this - terminal blocks, lugs, other good stuff, the type of stuff they use to wire a circuit breaker panel.
A test for an HCl drip with water will come very close. I'd have it drip as far away from the electrodes as possible, and let natural circulation
diffuse it throughout your cell.
If I understand your post correctly, you have a 3.3V power supply? If so you are correct, that is a bit on the low side. Power supplies can be used
in series with little problem, so two would be 6.6V, a tad high for chlorate, but not bad. I'd NOT use the lamp dimmer - they are designed for
115VAC. not 6.6VDC power. I'd simply open up the spacing between electrodes a bit, make it 50 to 75mm. Do you have an ammeter? If so, fill a beaker
with salt solution, power up, and find a spacing that will give you the amperage you desire. A 2L cell is pretty small and will heat rapidly with
anything much above 20 amps. You can immerse the bottle in a larger container 3/4 full of water, and position a fan to blow across the water surface,
and it will do an effective job to chill the cell. At 10 or 15 amps, it might be OK, but I'd still place the cell in a bucket or something in case of
a structural failure - the bucket catches the corrosive liquor.
HTH, good luck, snap a few pics!
|
|
|
Bikemaster
Hazard to Others
  
Posts: 120
Registered: 8-10-2008
Member Is Offline
Mood: No Mood
|
|
I = V/R.... i fell very stupid...
i made over 6 mounth that my cell run and i was not thinking about this...
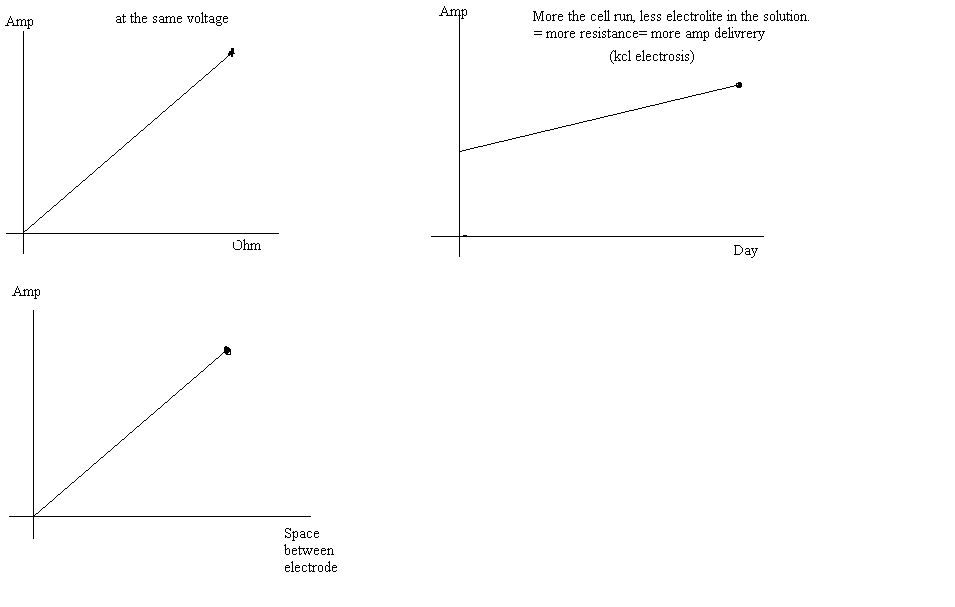
If follow you, all in these graph is real???
So the the nacl electrolise can give a better yeild because the resistance is always the same?(never precipate) because with the kcl electrosis the
resistance increase slowly so we can't put the cell to the max amp possible when we start the cell because the resistance will become to big and the
current will no more pass.
thank a lot for those think because it will help a lot . i was not thinking about
the size of the wire too... . i was not thinking about
the size of the wire too...
no i don't have this power supply (400A 3.3v) it was just a question tu now how to control the number of amp passing in the cell. It was just dann2
that want to but this power supply. In my futur cell, i will have approx 100 amps divided on three 2x6 inchs mmo mesh anode. I will probably use
computer psu.
I will take picture of the cell contruction, but i want to get the most info as possible before to make it and do full of error. I will probably seed
pic of my homemade ph control in less than one week.
[Edite le 16-3-2009 par Bikemaster]
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello Folks,
@BM
You have the Ohms law the wrong way around in your head!!
V = Volts
I = Current
R = Resistance
V = I * R
I = V/R
R = V/I
Look on the cell as a resistor.
If we increase the Voltage accross the cell the Current gets higher.
Decrease V and I goes down.
You can also say that if you increase/(decrease) Current (I) then V will go up/(down).
Unfortunately the 'resistor' (the cell) we are talking about here does not hold its
resistance value steady. It varies in resistance as temperature, concentration of products, electrode spacing etc change, so that when we connect a
power supply that has a CONSTANT
output voltage (most power supplies are like this when used in their normal range) to the cell the Voltage will stay
steady accross cell but the current will decrease/(increase) as the resistance increases/(decreases). The Voltage will stay steady because that is the
type of power supply that is connected to the cell, a constant Voltage supply also called a Voltage regulated power supply.
When you have a constant current power supply the output current stays at the amount that you have set at the controlls of the supply and the voltage
varies in sympathy with the resistance. That is, the Voltage will increase/(decrease) as the resistance increases/(decreases). The current into cell
stays steady. This is the type of power supply we desire for out chlorate cells.
Read up on Ohms law.
To complicate matters further some supplies are a bit of both (Voltage may vary a bit and current will vary a bit). This is what starts to happen if
you use a resistor + a constant Voltage supply to power the cell. Lets forget about this further complication for now though.
The Current (I) (going into cell) is the RATE of flow of electrons. One amp flowing for 26.802 hours will move (put into cell) one mole of electrons.
Read up on moles!!
Some info here
About the dimmer. Are you going to put it on the input of the power supply? It would not work as the supply (link way above) would not function on the
waveform coming from a dimmer.
@Swede.
There is a link to a large (constant voltage unfortunately) power supply just above graph above that Bikemaster is referring to.
Dann2
[Edited on 16-3-2009 by dann2]
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
@Dann2, you beat me by moments with that post!
Bikemaster, You've got it backwards a bit - Resistance is the denominator in I = V/R, meaning twice the resistance = 1/2 the current!  If you got a better yield than two separate identical systems running at the same
time, then something else is at work. If you got a better yield than two separate identical systems running at the same
time, then something else is at work.
With a (per)chlorate cell there are some fairly simple rules of thumb, and some that are not so simple. With all else being equal, and voltage fixed,
and assuming an oversized power supply like our imaginary 5V 1,000 Amp supply...
1) Bigger Electrodes = more amperage
2) Closer spacing = more amperage
3) Higher ionic species concentration = more amperage
4) Heaver cabling and electrode shanks = more amperage, because light cabling causes a voltage loss over a given run, and the longer the cables are,
the more loss you have. If you hooked two very long, light wires to your cell and turned it on, then measured the voltage at the electrode shanks,
you might measure 4.23. Replace the light wires with short, heavy cables, and repeat, you might measure 4.85. According to I=V/R, if the voltage
increases, so does the current. Heavier cabling has less resistance than lighter cabling, and less resistance means more current will flow at a given
voltage.
Any time you have heating in your cabling and electrode straps, it's just wasted energy. Barely warm to the touch = good. It's trickier to keep
electrode straps cooler because they are usually Ti, and as a conductor, Ti is pretty lame, and heats up, whereas Cu would not.
With potassium salts, as the chlorate falls out, you really don't lose a lot of current, as the liquor is still a wild hash of ionic species like
chlorides, chlorate, hypochlorite, and others, and electricity likes aqueous ions... they help current flow. But you do lose some.
It might help you to try some numbers on paper with ohm's law, and do a bit of algebra, and you'll understand the relationship better. You'll get the
hang of it. For example, I have no idea of the resistance of my cell at the start, because I have never put an ohmmeter across it, but I can figure
it out because I know the other two variables, voltage, and current.
There are three simple variations on ohm's law. We've seen I = V/R. There is also
R=V/I and V = R x I
I set 5 V on my supply and get 45 amps. The resistance is then:
R = V/I
R=5/45
R = 0.111 ohms
If I put two of these cells in series, the resistance would double to 0.22 ohms. Solving for current:
I = V/R
I = 5/0.222
I = 22.5 Amps
Thus the current is cut in half with a fixed 5V supply. Don't get discouraged, the relationship between voltage, resistance, current, and power
(watts) can be tricky and confusing.
[Edited on 16-3-2009 by Swede]
|
|
|
Bikemaster
Hazard to Others
  
Posts: 120
Registered: 8-10-2008
Member Is Offline
Mood: No Mood
|
|
oups... all wrong i need to get a good refresh in electric law.
I saw those law 3 year ago and i think that forget some stuff...
For the chemistry part i dont have any probleme... i know what a mole is...
Ok now with the good law: I=V/R
exemple: power supply 15v 3A
I=V/R
3=15/R
3R=15
R=5 ohm
So i need a resistance of 5 ohm to get 3 amp
too much resistance ex:
I=15/30
I=0,5 Amp
smaller resistance ex:
I= 15/2,5
I= 6 amp
but the max amp than we can deliver is 3 amp so it only give 3 amp.
I expect to be to be ok...
i will have have electric and magnetique next session at school so i will be better in electricity
last thing, i need a multimetre,do this one can be good??? wach the spec not the color
http://cgi.ebay.com/ws/eBayISAPI.dll?ViewItem&item=39001...
i was writing during your post, i don't use 14 gauge wire, i use 22 (speaker cable) and the end these wire have turn to black and the plastic have
melt... can be a big lost. i think that i need to get good wire for my next cell.
O wait i have aluminium 6 gauge wire, use for give the energie to a welder, will it be ok.
[Edite le 16-3-2009 par Bikemaster]
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello,
Sorry about that Swede!
The 'resistance' of the cell is something you cannot really put a figure on as such. If you have a (purchased) resistor, say 20 ohms, it will stay
rock steady at 20 ohms (so long as you do not abuse it) as the current and voltage vary. Double Voltage, will double current. Increase Voltag by 10%,
current will increase by 10% etc. The resistor is linear, or at lease we are going to use it in its linear region of operation. This is how you use
the vast majority of resistors in electonic circuits.
The cell is a beast. Say at 1.4 volts you measure 0.4 amps accross cell, then the resistance of the cell is 3.5 Ohms.
If you increase the voltage by four you will NOT get four times the current. I don't know what you will get but it will be far far more than four *
0.4. Current might increase by 20 amps!. The resistance of the cell is nonlinear.
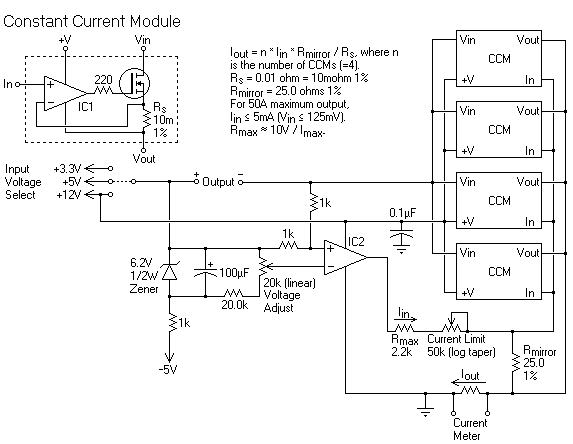
Try and obtain a constant Current supply, it simplifys things greatly.
I have been getting around to building the Constant Current module shown below (top left of diagram) but have never actually done it. It would be a
gread add on for a computer power supply.
There is another wolloper of a supply on ebay.uk here. May be worht the price it is constant voltage out though.
Dann2
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello,
The picture above is from 12AX7 (this board) btw.
Attached is some more info if anyone wants to build CC stuff onto Computer power supplies.
@BikeMaster. Remember if you go above the current rating of a constant voltage supply (usual type of supply) you will heat it up and it will cut off
or perhaps fail (or worse).
Dann2
Attachment: constant currents.pdf (51kB)
This file has been downloaded 565 times
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
Dann2 is correct, a cell is a living creature, with toxic waste products and a mind all its own, sometimes, but the basic Ohm's law will take you far
and is a powerful tool.
If there is one thing someone setting up a cell should invest in, and spend a lot of time searching for, it is a good power supply. It will save you
from more headaches than you'd ever imagine. A fixed supply, or a computer supply, is fine for casual experiments, but if you want production, at a
minimum I'd recommend a variable supply capable of at least 25 amps... 50 to 100 is better, and the supply should be variable from 0 to 10 (or more)
Volts. Linear or switching, it doesn't matter, and the switching supplies are 1/5 the weight and bulk of a linear... and they are cheaper.
MOST reasonably modern supplies that are variable have CC built into the device. A typical eBay ad would say "CC/CV Operation" which is what we want.
When I first started, I haunted eBay and bought a 100 amp 0-10V supply for $25. Shipping was $75, because it was linear and weighed a ton. A few
weeks later, I snagged a 0-10V, 0-60A supply for $75, a switching supply with digital readouts. Sweet deal. They are out there, it simply requires
patience. Used supplies like these normally would sell for hundreds of dollars. But the beauty of them is that they can operate a cell that is the
size of a 100 ml beaker, OR a giant 10 liter cell running at 60 amps. One good power supply, and you are set.
I always try to operate mine at no more than 75% rated amperage, and one thing to remember, if your shop gets to 35 or 40 degrees C (HOT!) then the
supplies must be "derated", meaning they cannot and will not supply their rated power. My linear supply gets fairly hot. I bought a pair of cheap
fans and set them up to blow air through the case, which really helps.
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
I forgot to add, your 6 gauge Al wire will probably be fine, just be sure to abrade (sandpaper) the aluminum at the connection points, and make sure
they are TIGHT. Al wire tends to form oxide skins that inhibit current flow at the connection points.
The best cabling I've ever found is Cu welding cable. Try a welding shop... it is sold by the foot. They will have Cu cable from #6 and bigger, and
the beauty of welding cable is that the Cu strands are fine; it is very flexible, and the coating is soft rubber, unlike household #4 which is REALLY
stiff and coated with inflexible PVC plastic. Well worth pursuing. #4 cable cost me $2 per foot at a local store, and 12 feet is adequate for a
large setup.
[Edited on 17-3-2009 by Swede]
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
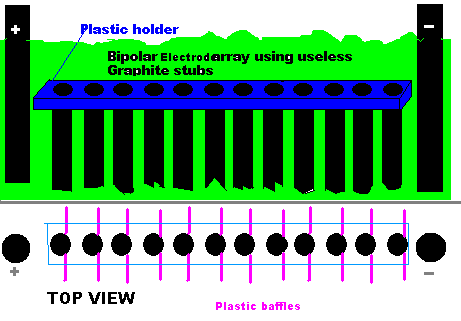
Hello Folks,
A guy asked me some time ago via u2u what to do with a heap of Graphite stubs that he could not use. (Sorry I don't remember who but I rubbed out my
u2u's.)
Check out below. You could make a Bipolar Electrode array from them. There is no need to actually connect power to the stubs as the current 'jumps'
the gap with each stub being an Anode on one side and a Cathode the other side.
You could just hang the stubs from the lid of the cell with cord that would withstand the Chlorate cell conditions if you though the plastic holder
was too much bother to make.
For every 6 grams of Graphite stubs you have one KG of Chlorate. (with pH controll).
You can see stuff about the Bipolar electrode array up this thread at posts around 25 and 26 Oct. 2008
Dann2
LONG LIVE THE PAUPERS MMO
[Edited on 19-3-2009 by dann2]
|
|
|
| Pages:
1
..
22
23
24
25
26
..
48 |
|