| Pages:
1
..
23
24
25
26
27
..
48 |
Aqua_Fortis_100%
Hazard to Others
  
Posts: 302
Registered: 24-12-2006
Location: Brazil
Member Is Offline
Mood: †
|
|
The guy was me 
Dann2, thanks for that great idea, I still have plenty of that stubs from previous cells and this week I discovered a HUGE PILE of graphite pieces
(probably sintered, some cm long, since it was on metalurgical engineering park on university ) ; collected some hundred grams (several pieces) to
future testing, probably worth eforth using in that scheme, although they are somewhat irregular pieces.
Only hope the electrical resistance to drop reasonably well with this bipolar scheme to give good currents (+5V PC PSU).
[Edited on 19-3-2009 by Aqua_Fortis_100%]
"The secret of freedom lies in educating people, whereas the secret of tyranny is in keeping them ignorant."
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello,
I had not though aboutt the voltage accross the array of Electrodes. Perhaps my simple scheme will not work. I fact I don't think it will unless you
put plastic baffles between each electrode (see picture last post). This is starting to get awkward and a lot of work. The bipolar electrodes in the
pictures are flat sheets. They may be put into a cell where each electrode actually goes from wall to wall and from top of liqued to bottom of the
cell?
Cheers.
Dann2
|
|
|
chief
National Hazard
   
Posts: 630
Registered: 19-7-2007
Member Is Offline
Mood: No Mood
|
|
The bipolar idea I like ! It could be altered like this:
==> use a lot of crushed graphite-pieces in some sort of emulsion, which means: Constantly stirring it with some drilling engine, a mixer attached
(such as for mixing concrete, 1.50 bucks at the hardware-store) ...
Maybe this method could even be used with the microscopic powder from eroded anodes ... ? The voltage would be somehow divided , as shielded by the
many graphite-particles ... ; maybe it would drop below a useful value for the sub-current-paths ? Would it still create chlorate ? Or would this be a
novel way to lead current through an electrolyte without electrolyzing it ?
==< But the electrolyte would have to have a no-better conductivity than the carbon, so that the current would really prefer it to be split like
this ...
Also: PbO2-platelets could be used .., since the current path through such a platelet would be geometrically much shorter than around it and through
the electrolyte ...
As I understand: With the model depicted in the post above the voltage would have to be increased to the multitude-value of multiplication by the
electrode-count ...
[Edited on 19-3-2009 by chief]
[Edited on 19-3-2009 by chief]
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello
I altered the gif I posted to include baffles.
I can't see the Graphite/PbO2 powdered thing working. It would all go into suspension would it not.
Cheers,
Dann2
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by dann2
A guy asked me some time ago via u2u what to do with a heap of Graphite stubs that he could not use. [...] You could make a Bipolar Electrode array
from them. |
How do the galvanic potentials affect the drive voltage in such a cell? I don't know what the
anode and cathode potentials for graphite-in-a-chlorate-cell are.
|
|
|
Aqua_Fortis_100%
Hazard to Others
  
Posts: 302
Registered: 24-12-2006
Location: Brazil
Member Is Offline
Mood: †
|
|
Thanks Dann2
Unfortunatelly I have just stubs from gouging rods and another from irregular pieces; if they cant be used on bipolar scheme then it may be really
just waste (or then crushed as source of carbon for another projects like some homemade castable refractory mix or maybe as reactant)..
Maybe the stubs may be 'glued' in so way they form anything that resemble sheets, but I have a feel that will be hassle and probably not work, but
anyways, I may be wrong..
"The secret of freedom lies in educating people, whereas the secret of tyranny is in keeping them ignorant."
|
|
|
Bikemaster
Hazard to Others
  
Posts: 120
Registered: 8-10-2008
Member Is Offline
Mood: No Mood
|
|
My ph control setting is now working
I start from the idea of Dann2 to make it but i change some stuff cause it has a problem in his setting. The first idea was to put a bottle link with
a tube to a small tip. But the problem of this setting was that when the level of water go down, the droping rate was going down too... So to get a
constant droping rate, the level of water need to always be the same. To more easily understand the setting, i post some pic-->.
With some calculation, i find that i need 1 drop every 12 sec to get 13,4 ml per hour, wicth is what i need for my 100Amp chlorate cell.
I will try this ph control systheme when my cell were be finish, so it is maybe be ready in one or two month...
The last thing, swede, i search for the kind of clip to put at the end of the wire to plug to the anode and the cathode, but i the biggest size that i
find was for 12 gauge wire... So i made from copper tubing, will it be ok? Also, thank for helping me for the PID controller setting.



[Edite le 21-3-2009 par Bikemaster]
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello,
@BikeMaster
Thats a good idea. It is like one of those water despensors for chickens (!) that keep the tray filled whenever the level drops a bit.
If we has some easy/cheap way to monitor pH.........
Also is it possible for you to edit that last post and make the last picture more narrow. It makes all the text go off my screen and drives me nuts.
It is too wide for the board in general.
Thanks,
Dann2
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
@Bikemaster - all looks good! Very clever arrangement! Just realize that the portion of the HCl rig open to the atmosphere will vent a bit of HCl
fumes. If there is any ferrous metal in the vicinity, it will soon corrode. But nicely executed using components that are essentially free. The copper
looks fine, too. There are a hundred ways to attach heavy cabling to electrodes, all of which will work, and I'm sure yours will too. When it's
running, check for heat. Most of the heat generated in a high-amperage setup comes from the titanium straps' resistance, and the connector both helps
the current along, AND acts as a heat sink. Cu conducts heat nicely, and the cables themselves will help carry the heat away.
It looks like the bug has bit yet another poor (per)chlorate victim! 
To all interested: The LD anode(s) that I plated in January are still untested, but I have finalized my LD paper, "Lead Dioxide for Dummies." It
includes both the research and the experimental portions of this whole thing, although no real conclusions - yet. I will add those when I test the
anodes.
The download is 1.1 megabytes due to pics. Guaranteed virus-free, hot off the press.
http://www.5bears.com/ld/ldfd01.doc
Even if this particular method does not work, I think guys may find the information helpful. Good luck to all!
[Edited on 21-3-2009 by Swede]
|
|
|
Bikemaster
Hazard to Others
  
Posts: 120
Registered: 8-10-2008
Member Is Offline
Mood: No Mood
|
|
For the hcl fume, it is not a big probleme because my setting will be outside and the open container will be at least one foot from any metal. the
only think than i will change on this setting, it the top bottle. i will use a more rigide one because the pressure press on it when the water want to
go down..
i just have to make somethink to hold this setting and it will be ready to use... (don"t want to use my clamps for this...)
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
Bikemaster, a suggestion - you've seen hospital IV drip systems... they have a clamp arrangement on the soft tube to vary the flow. A tubing clamp
could easily be engineered, given what you made with the copper. Some sort of threaded channel, with the rounded end of a screw bearing on the tube.
I know you are looking for constant drip. If you use a tubing clamp, and set the source high enough, the variation in drip is going to be REALLY
small, small enough so that you could probably ignore it and just use the average. These systems do not require super-precise HCl additions. Just get
it and keep it between 6.0 and 7.4, and you're in the ballpark. 6.4 to 6.8, even better. Just a thought, it might be worth simplifying it. And you
could go with a siphon. Vent that top bottle, set it upright, and start the siphon, you'll be good to go.
Good luck!
|
|
|
Bikemaster
Hazard to Others
  
Posts: 120
Registered: 8-10-2008
Member Is Offline
Mood: No Mood
|
|
hey swede,
I read your research report, Good job, it will help me went i will try to make perchlorate one day. but i find just something bizare, every were that
we see LD plating, they say never go over 55 C??? but your plating bath was at 65 C.
And do the volume of the plating bath will really make a big difference. cauze if i try one day to make a LD anode, i think that it will be on a small
graphite rod (lantern baterie one) and i will not make a big bath for this, will it be ok with a 1 liter solution.
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
Bikemaster, pretty much every patent I read recommended between 60 and 80 degrees. I'd be interested in any additional resources that would shed
light on the temperature issue. Are you sure you're not thinking of 55 degrees for a graphite anode in a chlorate cell? There, if I remember, lower
temps are better as you have less erosion.
The reason a large LD bath is generally better is because the chemistry of the working bath changes rapidly; HNO3 evolves, Pb ions are depleted, and
if both are not kept in a proper range, wrecks the plate job. A large bath, by definition, adds thermal and chemical stability. Bad stuff just can't
happen as fast.
On the other hand, if your anode is exceptionally small (just a test) you can use a smaller bath. I personally wouldn't try it with less than a
liter, though, but there's no hard and fast rule. This is just a case where "Bigger IS better" but also much more expensive, and more of a pain to
set up.
|
|
|
Gamal
Harmless

Posts: 24
Registered: 6-11-2005
Location: Sweden
Member Is Offline
Mood: No Mood
|
|
I have been following this thread for a while now, and I think it's time to enter in.
I was visiting a local producer of MMO-electrodes today. It was interesting to see the process a little closer. Ofcourse I asked to buy some
MMo-sheets (that was really my main reason for the visit ). He told me that I
couldn't as it would be much to expensive, he had to put up an order and all that. The only possible way to go was giving me some spillover. I left
with 3.5 kg of Ti gr1 and MMO-sheet metal ). He told me that I
couldn't as it would be much to expensive, he had to put up an order and all that. The only possible way to go was giving me some spillover. I left
with 3.5 kg of Ti gr1 and MMO-sheet metal 
I suppose sheet is better then mesh, right?
Now it's time to start working on the first test cell. I will make it simple, maybe with some form of ph control, current limiting and a PC power
supply. Some kind of glass jar (just have to eat the jam first) will do as a container.
I post my successes (or whatever) when I get something setup.
|
|
|
Bikemaster
Hazard to Others
  
Posts: 120
Registered: 8-10-2008
Member Is Offline
Mood: No Mood
|
|
Maybe the temperature will change for differente substrack... i read more on GSLD, so for plating graphite the temperature need to be under 55 C. but
for MMO it seem to be more.
For the solution size, the only reason that i want to make smaller size, is for the price. but can we re use the solution for more that one plating???
It will be a good way to save chemical and money.
Did you really get 3.5 kg of titanium and MMO . WoW! . WoW!
If it is possible, can i buy some??? like a small sheet of 2x6 of mmo and a 6x8 sheet of titanum
good luck for your futur cell
|
|
|
Gamal
Harmless

Posts: 24
Registered: 6-11-2005
Location: Sweden
Member Is Offline
Mood: No Mood
|
|
Bikemaster
I'm living in Sweden and I know the shipping cost would be quite high. For now I will keep my Ti and MMO until I know how mush I need in the future.
Try the cheep Ebay MMO mesh. I can PM you the address to the seller if you want it.
/Gamal
|
|
|
Bikemaster
Hazard to Others
  
Posts: 120
Registered: 8-10-2008
Member Is Offline
Mood: No Mood
|
|
I have 3 of the ebay <cheap> mmo anode. Personaly i am not thinking that is really cheap because i think that we pay a lot cause the anode is
ready to be use. (handle, perfectly coated and because it is the only seller on ebay). For those anode (2x6), i pay 80$ each and if i want to buy some
today it will cost me 100$ because of the canadian money rate...
I don't know how much you pay your mmo and titanium plate but i am pready sure that it is less that 100$can for each (2x6).
For the shipping price, i go on (Royal mail Airmail) and the shipping price don'tseem to be a lot
If you want to keep your titanium and your mmo, it is perfectly your choice but i just thinking of this because if you give me the adress of the
seller i can not buy 3,5 kg too and the price of the titanium and the mmo will be bigger... but if the price of these stuff seem to be good, send me
the adress and i will try to order some stuff.
thx
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
For LD plating, I have no idea how the eBay material will work. The anodes I plated in January were a different brand, and cut from a full sheet I
purchased. MMO formulations are application-specific. Pool chlorinators, chlorine generators, chlorates, and general chlor-alkali processes, all
seem to have different formulations, most of them a trade secret.
We know the eBay MMO makes chlorate, but beyond that, I have no idea. It seems to be good, sturdy stuff, and may plate nicely, but I need to set my
rig up again, which I was planning on doing anyhow very soon. I will try a small piece of the eBay MMO.
My new goal is a more modest coating of LD rather than the mass that plated on my Anode #2. Before I do that, I need to check the bath for Pb++
concentration and a few other things.
Overall, though, it seems that MMO is becoming more and more available... sorry dann2, the days of graphite as the primary tool for home chlorate
production appear numbered! 
Gamal, nice score. There is NO substitute for contacts in the industry, legwork, or both.
[Edited on 24-3-2009 by Swede]
|
|
|
Gamal
Harmless

Posts: 24
Registered: 6-11-2005
Location: Sweden
Member Is Offline
Mood: No Mood
|
|
This is a link to an expired action. The seller is laserred.
http://cgi.ebay.com/ws/eBayISAPI.dll?ViewItem&item=33030...
The mesh is 3" x 30" (4" inkl. the bent side wich can be used to make connections) for $9.99. That has to be cheap! You can ask him to put up an
auction for it.
Obviously, this hasn't been used in electrolysis, because of the shape, but it seem to work well when reading this tread. You could get some Ti sheet
metal if you contact some kind of engineering workshop in your neighbourhood. I think it's commonly used.
|
|
|
Gamal
Harmless

Posts: 24
Registered: 6-11-2005
Location: Sweden
Member Is Offline
Mood: No Mood
|
|
I found a patent describing a continous process from sodium chloride to potassium chlorate.
http://www.freepatentsonline.com/5087334.html
Allso have a look at the pdf there.
It's allways an interesting challenge to make processes run automatically.
I would like to go for the direct electrolysis of KCl. The problem here, as we know, is the formation of chrystals in the wrong places. What has to be
controlled there is the concentration and temp of the solution. With some simple mathematics I think it's possible to work out a contignous process
where those parametres is under good control. The heet from the cell should be enough as the only heet source. With some insulation around pumps and
hoses the chrystals should participate out at the right place I think.
Swede, you have been using some tubes used in plumbing in your cells. Does it work well? Is it standard plumbing plastic or some special ordered tubes
and gaskets. I've been thinking of using gasket silicon instead of the standard gaskets. Do you think it would work.
It would be interesting to see a description of your point welder. It was a nice pice of work. I like homemade tools!
Is it recommended to have a catode on each side of the MMO anode?
If the anode-catode is placed on one side of the anode, shouldn't that cause self circulating in the cell, especially when using high current? I think
that would be very positive for the reactions in the electrolyte.
[Edited on 24-3-2009 by Gamal]
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello,
That was a delicious piece of spillover! I reckon that you shook your fist at him!!
What do they normally do with their spillover (scrap pieces?). If you can get your hands on the stuff for a good price every month or so, you could
have a great ebay thing going on selling MMO sheet. (and doing the poor beleaguered Chlorate making community a great service too).
@Swede
I weighed a gouging rod today. 90 grams, it's 13mm diameter. If you take 15% off this for connections and the fact that the last small slivers will
fall down to the cell bottom and not be available for making Chlorate you are left with 76.5 grams. At 10 grams wear per Kg Chlorate (you can get 6
grams wear with EDM Graphite) that equates to 7.5 Kilo of Chlorate per Gouging rod!!!!!!!!!!!!!!!! Yeeeeeeeeeeee Haaaaaaaaaaaaaa   
BLESSED BE THE GOUGING ROD AND ALL IT'S WORKS
LONG LIVE THE POOR MAN'S MMO
Moving on to less serious things I plugged a 'killawatt' meter between the mains supply and my setup. Cell runs at 7 amps and it's a computer supply.
It it burning up 62 watts. Thats one unit every 16.1 hours = 1.5 units per day = 24 cents per day = 720 cents per month. I was surprised how much it
actually is. Most of the power is going on the supply. The cell is only burning up approx. 27 watts.
EDIT: Forgot to mention that I have a piece of hot Nicrome wire between cell and supply.
If you are searching for Titanium in Europe try the German ebay at:
http://www.ebay.de/
Search for Titan blech (titanium sheet)
Search for Titan stab (titanium rod)
Anyone know the German for Titanium mesh?
I have no German. Use the Alta Vista translaters if you have to read the descriptions though they are usually self explanatory. Make sure the seller
does PayPal. Bank transfers are a pain.
Still running my cell with the Poor Man's Platinum (Lead Dioxide). There is little or no erosion on the LD. Guess it is due to the pH controll though
it is early days for the cell yet. It has not reached the 'difficult' zone yet (low Chloride concentration).
I wonder since there has been a terrible failure rate on Graphite Substrate LD Anodes would it have been the fact that they were always being used in
a non pH controlled cell. If pH was controlled they would probably have faired better.
Dann2
[Edited on 24-3-2009 by dann2]
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
Haha, dann2, you are a dogged advocate of graphite!  While you are decanting
and getting ready for a decomp to potassium, I am washing masses of snow-white potassium chlorate crystals. MMO is the King of Chlorate! While you are decanting
and getting ready for a decomp to potassium, I am washing masses of snow-white potassium chlorate crystals. MMO is the King of Chlorate!
Gamal, last fall I attempted an elaborate 2-cell pumped system, which I called the "T-Cell". The system used a hot "Electrode Cell" (EC) and a cool
"Collection Chamber", the CC. I used Potassium salts.
If you're interested, I blogged the whole experience; I don't know if you've seen it or not: Starting about here....
http://www.apcforum.net/forums/blog/swede/index.php?view=sho...
In summary, it was a disaster with potassium once the crystals began to form. Prior to crystal formation, it was working spectacularly. By varying
the pump rate, I could create a dT between the two cells of 20 to 40 degrees C. But once the system was saturated with chlorate, as the liquor
traveled through the tubes, it cooled almost instantly, and the tubes jammed hopelessly with crystals.
It was a huge effort that I am not ready to repeat at this time, mainly because a single, large cell works so well... I couldn't see the benefit of
circulation. But that doesn't mean it doesn't work. Commercial plants circulate (with sodium salts) or have a linear process, with multiple
chambers, and a saturated chlorate solution coming out the end of the line.
There are few plastics that would have a long life in a chlorate system. The big four that I am aware of, and that I know work:
(C)PVC
PVDF
PTFE
PET
For tubing, there are Tygon formulations that do work, but a better option is a wide-bore CPVC pipe system vs. flexible tubing.
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
Hello,
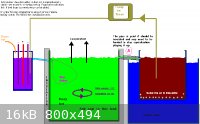
I may have posted this monstrosity here before but it is (IMHO) the 'de Nora' of all Chlorate cell designs!
If you are going for a crystallizer/collector you really need a miniumum of two large chambers or better to have three chambers if you are going for
pH controll.
The crystalization chamber needs to be cool.
The chemical Chlorate chamber (assuming pH control) is best hot, both for the small increase in efficiency and to stop the formed Chlorate coming out
of solution.
The actual chamber with the electrodes can be quite small in relation to the size of the Chemical Chlorate formation chamber. If not using pH controll
then two large chambers will be OK. One is the Chlorate cell, the other the crystallizer.
The problem point is Point A, as discovered by Swede.
A filter bag may be a great help if using the QUEEN[/COLOR] (did ya ever play Chess)
 of Chlorate Anodes. (pH controll definitely recommended). of Chlorate Anodes. (pH controll definitely recommended).
Point A will be a problem with either Na or K as you are going to be dealing with saturated solutions for both. It will just take longer for the Na
system to reach saturation.
Dann2
|
|
|
Swede
Hazard to Others
  
Posts: 491
Registered: 4-9-2008
Member Is Offline
Mood: No Mood
|
|
I like it. Sodium would absolutely be the way to go. If one has the ability to create such a beast, then one also has the knowledge of how to
process the goodies!
But dann2, you do realize the cost of such a system would far outweigh the cost of acquiring a proper anode for it! 
Graphite in that system would be like using spray paint to touch up a Mercedes! 
|
|
|
watson.fawkes
International Hazard
    
Posts: 2793
Registered: 16-8-2008
Member Is Offline
Mood: No Mood
|
|
Certain bulk crystallizers, as I understand it, use a single tank and a thermal gradient somewhere in it, say, marked by a heating plane and a cooling
plane, within which crystal formation takes place. A matched pair of heating and cooling planes could be created by a heat pump suitable designed for
the temperatures in question. In this case there's no pipe to clog; the entire width of the tank is the "pipe" connecting the two halves.
|
|
|
| Pages:
1
..
23
24
25
26
27
..
48 |