guy
National Hazard
   
Posts: 982
Registered: 14-4-2004
Location: California, USA
Member Is Offline
Mood: Catalytic!
|
|
Coordiate Covalent Bonding Questions
Coordination covalent bonding in Ni(CO)4 is very confusing to me. I have looked at many books and sources but I can't completely understand it. What
I want to know is how the bonding of the CO and Ni works.
From General Chemistry by Linus Pauling
"These bonds show that the Ni-C bond has a large amount of double-bond character and the C-O bond has a considerable amount of triple-bond
character...For these structures [SEE ATTACTHMENT] all nine of 3d<sup>5</sup>4s4p<sup>3</sup> orbitals of the nickel atom are
used, either for bond formation or occupancy by an unshared pair."
It says here that the bonds are double bonds.
Another quote from this siteprovides another explanation, BUT I on't see how this can fit in with the double bond explanation.
"Ni(CO)4:
1s<sup>2</sup>2s<sup>2</sup>2p<sup>6</sup>3s<sup>2</sup>3p<sup>6</sup>3d<sup>10</
sup>4s<sup>2</sup>4p<sup>6</sup> The bonding in this molecule uses the 4s and 4p orbitals on nickel to form a sp3
tetrahedral structure, filling these orbitals with electrons from carbon monoxide. The two electrons normally in the 4s orbital of nickel move to fill
the nickel 3d orbital, leaving the structure with no unpaired electrons."
If they all form sp<sup>3</sup> then they should all be single bonds.
Can anyone clarify this problem?
I am guessing that the orbitals hybridize to form d<sup>5</sup>sp<sup>3</sup>. All the orbitals have single electrons except
one (which have a pair). The CO moleules donate its electrons to the orbitals 2 at a time (for figure A). I can't figure out figure B, somehow there
are 2 orbitals with electron pairs and one empty orbital (explains single bond between CO and Ni molecule).
[Edited on 1/6/2006 by guy]
[Edited on 1/6/2006 by guy]
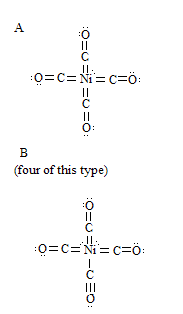
|
|
|
|