Electra
Hazard to Others
  
Posts: 179
Registered: 11-12-2013
Member Is Offline
Mood: No Mood
|
|
Finding a low-cost IR Spectrometer for reaction monitoring?
I've only searched briefly and most of the units I encountered were fairly expensive, but full of different features. Can anyone suggest a not too
pricy (not over $1000) IR spectrometer that I could use with some sort of probe attacahment to monitor reactions? I'm sure there's one out there I
just haven't found it yet. It doesn't even need to have fancy software to come with it. If I can get it to send its data to a port on my computer, I
can probably write a really easy program to record/monitor it.... I just need the IR-Spectroscopy part of it.
[Edited on 27-11-2014 by Electra]
|
|
|
smaerd
International Hazard
    
Posts: 1262
Registered: 23-1-2010
Member Is Offline
Mood: hmm...
|
|
I don't believe such a device is in existence as of yet. Aside from ATR and continuous flow techniques I'm not really aware of any insitu FT-IR
methodologies or 'probes'. Even used FT-IR's tend to be around the $1,000 mark unfortunately. It would be interesting to hear of anything other-wise.
|
|
|
smaerd
International Hazard
    
Posts: 1262
Registered: 23-1-2010
Member Is Offline
Mood: hmm...
|
|
Well my eyes have been opened to FT-IR/ATR Probes. not sure how applicable this might be to what you want to do but I thought it was cool and on topic
so check this paper out
http://www.remspec.com/pdfs/2703_o.pdf
Fiber-optic Probes for Mid-infrared Spectrometry. Peter J. Melling and Mary Thomson. Remspec Corporation, Sturbridge, MA, USA.
|
|
|
byko3y
National Hazard
   
Posts: 721
Registered: 16-3-2015
Member Is Offline
Mood: dooM
|
|
I was wondering why nobody tried to make a dispersive IR spectrometer using a diffraction grating? Using two gratings you can get resolution of 50
nm/mm (approx 30 cm-1/mm) at the distance of 100 mm for 3.5 um grating at the range of 5000-1500 cm-1 (2.0-6.6 um) and
resolution of 120 nm/mm (approx 12 cm-1/mm) at the same distance of 100 mm for 10.6 um grating at the range of 1700-510 cm-1
(5.9-19.7 um):
https://www.thorlabs.com/newgrouppage9.cfm?objectgroup_id=86...
4000-500 cm-1 is a perfect range for IR spectrometer, although the windows would be a problem (you don't want a dust in your grating), only
potassium bromide is capable of transmitting up to 400 cm-1.
https://www.edmundoptics.com/resources/application-notes/opt...
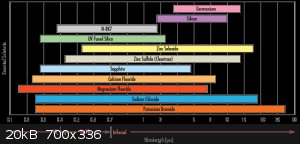
(there's also a caesium iodide, but it's a pain in the ass due to softness and hygroscopicity).

Interference spectrometer is much harder to make from scratch due to a very high precision required, especially beamsplitter: 1/4 wave - about 500 nm
in position and geometry in every transmitting and reflecting part until detector.
If you can make windows out of NaCl or KBr, then you could actually try to make a prism out of it. However, KBr prism in the shape of equilateral
triangle gives only 0.04 rad shift between 1 um and 10 nm ( http://www.janis.com/libraries/window_transmissions/potassiu... , correct me if my calculations are wrong ), thus wavelength resolution is 2000
nm/mm (approx 800 cm-1) at 100 mm distance from prism, which is much lower then diffraction grates provide, so it's much harder to get at
least 10 cm-1 resolution required for analysis, which also leads to high required optics' precision.
|
|
|
Tdep
National Hazard
   
Posts: 516
Registered: 31-1-2013
Location: Laser broken since Feb 2020 lol
Member Is Offline
Mood: PhD is done! It isn't good but it's over lol
|
|
In response to the original (old) question on low cost FT-IR spectrometers, while there aren't any now, in the next decade or two that could become a
possibility, not because FT-IR will get better, but I believe it will be superceded by new technology (and that gives the opportunity to pick up 'old'
tech at low cost... Well that's the thought).
What's the new tech?? Dual frequency comb lasers. It's still not quite here because Mid-IR lasers aren't all that easy but if it continues to be
developed the way it has been, it may replace FT-IR as a faster/smallermore reliable outcome. Easily field deployable, no moving parts. Read more
here: https://www.nature.com/articles/s41566-018-0114-7
Or maybe not, just some late night speculation
|
|
|
XeonTheMGPony
International Hazard
    
Posts: 1636
Registered: 5-1-2016
Member Is Offline
Mood: No Mood
|
|
well if any come across one in the 2K range it is one of the things I want to get to analyze any products I make for purity.
|
|
|
brubei
Hazard to Others
  
Posts: 187
Registered: 8-3-2015
Location: France
Member Is Offline
Mood: No Mood
|
|
Does this fit for you?
https://www.consumerphysics.com
[Edited on 27-5-2018 by brubei]
|
|
|
byko3y
National Hazard
   
Posts: 721
Registered: 16-3-2015
Member Is Offline
Mood: dooM
|
|
Quote: Originally posted by Tdep  | In response to the original (old) question on low cost FT-IR spectrometers, while there aren't any now, in the next decade or two that could become a
possibility, not because FT-IR will get better, but I believe it will be superceded by new technology (and that gives the opportunity to pick up 'old'
tech at low cost... Well that's the thought).
What's the new tech?? Dual frequency comb lasers. It's still not quite here because Mid-IR lasers aren't all that easy but if it continues to be
developed the way it has been, it may replace FT-IR as a faster/smallermore reliable outcome. Easily field deployable, no moving parts. Read more
here: https://www.nature.com/articles/s41566-018-0114-7
Or maybe not, just some late night speculation |
I didn't know about this technology, for those who are interested here's a review of basic principles: https://www.osapublishing.org/optica/abstract.cfm?URI=optica...
The largest problem with double-comb spectrometry is generating the combs: https://en.wikipedia.org/wiki/Frequency_comb
The price of a single-comb source is astronomical, and I can't even imagine the price of the synchronized dual-comb source.
Most likely when the technology becomes more widespread the prices would drop and the whole spectrometer would become relatively cheap, like 100-200k$
per unit. It can become a good tool for extremely precise measurement of spectrum. However, experimental FTIR spectrometers are capable of reaching
0.001 cm-1 precision, those are very expensive too though: https://doi.org/10.1016/j.jms.2015.03.004
Near-IR spectrometry is useless.
|
|
|
coppercone
Hazard to Others
  
Posts: 133
Registered: 5-5-2018
Member Is Offline
|
|
The conventional way would be to run a test reaction for a long period of time and take a buncha samples and freeze em quick then analyze them to make
a reaction timeline? Like stick the cuvettes in liquid nitrogen
Does that usually work? Or do you pretty much gotta run around like a chicken with your head cut off putting samples into the thing while you are
running a reaction?
[Edited on 27-5-2018 by coppercone]
[Edited on 27-5-2018 by coppercone]
|
|
|
ftirinih
Harmless

Posts: 27
Registered: 28-10-2007
Member Is Offline
Mood: No Mood
|
|
I've been very interested in FTIR for over a decade. The difficulty to getting one working is that you need 4 hard to find items:
1. FTIR unit - working
2. Interface card to couple FTIR to a computer
3. PC with appropriate ISA slot (BOMEM)
4. Software to collect inteferograms and produce spectra
In my collection I have a Bio-Rad FTS-40, a Nicolet 210 and Bomem MB series. The only one I ever got completely working was the Bomem. The FTS-40
needed clean compressed air to operate the air bearing, but the unit was not usable since the beamsplitter was ruined. The Nicolet I couldn't get to
work even after putting together all 4 of the items you need. The Bomem I had better luck with. They can be found on ebay, generally the seller asks
way too much. The software and interface card was almost impossible to find, but I did finally did get them.
|
|
|
byko3y
National Hazard
   
Posts: 721
Registered: 16-3-2015
Member Is Offline
Mood: dooM
|
|
I really feel like the final budget of your project is similar to the price of working unit.
|
|
|
ftirinih
Harmless

Posts: 27
Registered: 28-10-2007
Member Is Offline
Mood: No Mood
|
|
Here is what I spent:
1. Bomem MB155, complete and working. $250 +$65 shipping
2. Bomem SEQ51 ISA interface card. $65
3. Bomem FTIR Software $50
4. Gateway PC with ISA slot for SEQ51 card. $25
About $500 for a working system. It took patience and constant effort to find these with a great deal of luck. All from different sources.
A turnkey used Bomem system is ~$2-3,000 from what I found.
|
|
|
byko3y
National Hazard
   
Posts: 721
Registered: 16-3-2015
Member Is Offline
Mood: dooM
|
|
Dunno, https://www.ebay.com/itm/ABB-Bomen-MB104-FTLA2000-104-Arid-Z... - here's 1300$ for working one without software.
|
|
|
ftirinih
Harmless

Posts: 27
Registered: 28-10-2007
Member Is Offline
Mood: No Mood
|
|
That is a nice example. The MB104 has a non-hygroscopic window which is a good feature. The rub is that you have to scare up the interface card and
the software, very rare. These are usually discarded because the PC that runs the interferometer is an obsolete, worthless model and contains the
card and software.
In the ebay photos you are shown the 4 leds, power, zpd, scan and data. If it is working correctly only the power and data leds show be lit. Not
sure what state the instrument was in when the picture was taken.
I get the impression that you've never owned or operated an FTIR. Is that true?
|
|
|
byko3y
National Hazard
   
Posts: 721
Registered: 16-3-2015
Member Is Offline
Mood: dooM
|
|
You are correct, I've never operated the FTIR.
I can see DB-25 parallel port on the MB104 and I have PC for it.
The problem is that still the overall cost of device would be equal to the cost of some cheap used car in my region. And also I have no chances of
accessing NMR device.
MB104
Internal source: SiC
Optics: ZnSe
Detector: DTGS ZnSe window
Range: 6,500-500 cm-1
MB155S
Internal source: dual SiC / NIR
Optics: KCl
Detector: MIR-NIR DTGS
Range: 12,000-450 cm-1
Sinc selenide can't be used past 550 cm-1, I really doubt the true range of MB104 spans to 500 cm-1.
|
|
|
ftirinih
Harmless

Posts: 27
Registered: 28-10-2007
Member Is Offline
Mood: No Mood
|
|
The glass is more than half full. I posted to let people know that it is possible to bring up a working FTIR and what my particular experience has
been. That is valuable to anyone considering such an endeavor.
|
|
|
Met101
Harmless

Posts: 1
Registered: 22-1-2019
Member Is Offline
|
|
FTIR Software
Have acquired an MB155S, but like you have mentioned no software. If there is a source for software I would like to find it. Any info would be
helpful.
|
|
|
beerwiz
Hazard to Others
  
Posts: 128
Registered: 6-2-2014
Member Is Offline
Mood: No Mood
|
|
As far as I know IR spectrometers only work on pure compounds and can't be used with samples of mixtures of compounds (more than 1 compound).
Furthermore, I can't imagine using it to test a reaction solution as the solvent(s) will interfere with the reading not to mention the mixture of
compounds in the solution.
|
|
|
CharlieA
National Hazard
   
Posts: 645
Registered: 11-8-2015
Location: Missouri, USA
Member Is Offline
Mood: No Mood
|
|
If a pure substance is diluted in various proportions with an inert substance, the intensities of the characteristic absorption bands will be
proportional to the amount of substance present, and likewise for a mixture of components (e.g., a mixture containing unreacted starting materials,
perhaps intermediates if any, and product(s). The course of the reaction can be followed by periodically taking samples of the reaction mix and
"quenching" the reaction (for example, by dilution or neutralization, or other method). The intensities of the absorption bands in the spectra of
these aliquots will vary with the amounts of the various substances present.
|
|
|
beerwiz
Hazard to Others
  
Posts: 128
Registered: 6-2-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by CharlieA  | | If a pure substance is diluted in various proportions with an inert substance, the intensities of the characteristic absorption bands will be
proportional to the amount of substance present, and likewise for a mixture of components (e.g., a mixture containing unreacted starting materials,
perhaps intermediates if any, and product(s). The course of the reaction can be followed by periodically taking samples of the reaction mix and
"quenching" the reaction (for example, by dilution or neutralization, or other method). The intensities of the absorption bands in the spectra of
these aliquots will vary with the amounts of the various substances present. |
How would you do this? Sounds like science fiction to me.
The only practical way to make FTIR work for reaction monitoring is to periodically take samples and test with TLC on a silver iodide TLC plate. If
you are lucky and can visualize the spots, scrape them off and run the IR on them. Silver Iodide is transparent to IR so only the pure substance will
be analyzed.
If you can't visualize the spots by UV 254 or 360 then run it on a silica TLC plate with UV254 indicator and scrape off the spots you see under UV254.
Then extract and filter with a suitable solvent using a pipette with a piece of cotton as filter or use a syringe with filter. Run the IR on the
sample liquid subtracting the solvent. It's best if you can evaporate the liquid on the ATR (with a blowdryer) and test the residue, solvent
subtraction never worked for me well.
[Edited on 25-1-2019 by beerwiz]
|
|
|
Ubya
International Hazard
    
Posts: 1232
Registered: 23-11-2017
Location: Rome-Italy
Member Is Offline
Mood: I'm a maddo scientisto!!!
|
|
spectroscopy can look like science fiction  nature is not that simple. @beerwiz
the method you proposed is an option but not "the only practical way", by making subtraction spectra you can visualize the new bonds or the
disappearing of bonds without really doing anything to the sample, even if the reaction solvent absorbs IR (like water) if you subtract the spectra
from one taken before you will not see it, just the new bonds nature is not that simple. @beerwiz
the method you proposed is an option but not "the only practical way", by making subtraction spectra you can visualize the new bonds or the
disappearing of bonds without really doing anything to the sample, even if the reaction solvent absorbs IR (like water) if you subtract the spectra
from one taken before you will not see it, just the new bonds
---------------------------------------------------------------------
feel free to correct my grammar, or any mistakes i make
---------------------------------------------------------------------
|
|
|
CharlieA
National Hazard
   
Posts: 645
Registered: 11-8-2015
Location: Missouri, USA
Member Is Offline
Mood: No Mood
|
|
Ubya: Thank you. Charlie
|
|
|