deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Energetic dendrimers: cyanuric chloride + TRIS, followed by nitration
I had an idea for a hypothetical energetic dendrimer that I hope would have excellent explosive properties, e.g. insensitivity and good explosive
performance.
The idea is to use cyanuric chloride, a versatile and industrially available dendrimer forming reagent that reacts well with amines. An excellent
review is attached for your convenience and cited below (1).
Diethanolamine has been condensed with cyanuric chloride to form a water soluble polyol (2), so I propose coupling cyanuric chloride to three
equivalents of 2-Amino-2-hydroxymethyl-propane-1,3-diol (TRIS). TRIS is commonly used by microbiologists to produce buffer solutions.
This should result in a soluble polyol that could hypothetically be nitrated by conventional routes, precipitating the energetic dendrimer with a far
better OB than what could be formed from diethanolamine derivatives. Here is a simplified scheme of what I am proposing:
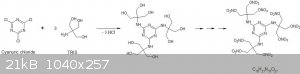
Typically, an organic base like DIPEA is employed to scavenge the HCl in such reactions, in a solvent like THF (3) or MeCN (4), however, DIPEA is
costly. I wonder if directly fusing three equivalents TRIS (m.p. 175°C) and cyanuric chloride (m.p. 154°C) could work... provided one safely deals
with the resulting copious amounts of noxious and dangerous HCl fumes evolved!
References (the first is attached for your convenience):
(1) Mackay B. Steffensen, et al. Dendrimers Based on [1,3,5]-Triazines. J Polym Sci Part A: Polym Chem 44: 3411–3433, 2006.
(2) Bansal, K.K., et al. Development and characterization of triazine based dendrimers for delivery of antitumor agent. J Nanosci Nanotechnol. 2010
Dec;10(12):8395-404.
(3) Abdellatif Chouai and Eric E. Simanek (2008). "Kilogram-Scale Synthesis of a Second-Generation Dendrimer Based on 1,3,5-Triazine Using Green and
Industrially Compatible Methods with a Single Chromatographic Step". J. Org. Chem. 73 (6): 2357–2366.
(4) WO application 03101980, "1,3,5-TRIAZINE DERIVATIVES AS LIGANDS FOR HUMAN ADENOSINE-A3 RECEPTORS", published 2003-12-11.
Attachment: Dendrimers Based on [1,3,5]-Triazines.pdf (1.2MB)
This file has been downloaded 673 times
EDIT: Added cyanuric chloride's melting point.
EDIT 2: Added the literature of diethanolamine/cyanuric chloride derived dendrimer.
[Edited on 5-12-2014 by deltaH]
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Upon nitration, the nitrogens are also nitrated to form nitramines 
R-NH-C(CH2OH)3 -HNO3-> R-N(NO2)-C(CH2ONO2)3
I had the same idea with tris and trichlorotrinitrobenzene...
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
| Quote: | Upon nitration, the nitrogens are also nitrated to form nitramines 
|
While I wish this would be true, I was under the impression that melamine resists nitration, AFAIK?
Interesting idea with the trichlorotrinitrobenzene and TRIS.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Melamine indead resist nitration to the third stage, but goes wel to mono and dinitramino compounds...sadly not much powerful than TNT.
But the story ils different for activated amino groups holding alkylic group...remember that 246-trinitroanilin can't be nitraminated (wel maybe in
anhydrous system with specific reactants)...but the methylamino brother does forme stable nitraminomethyl-trinitrobenzene ( tetryl)!
[Edited on 5-12-2014 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Point taken. Thanks for the excellent explanation.
Well then, this becomes quite the beast... I christen it Dendrimite 
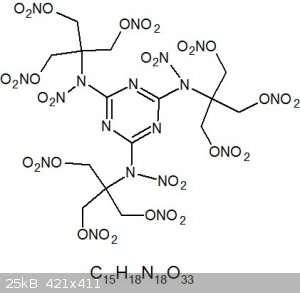
Any chance the nitro groups sit on the ring positions with the double bonds radiating outwards?
Anyhow, this thing doesn't look insensitive anymore, but at least it looks like it could pack quite a punch, perhaps similar to PETN?
Dendrimers tend to be good at absorbing other compounds, hence the drug delivery applications. Though dendrimite is a comparatively small dendrimer,
perhaps it may still absorb a little trinitroglycerine. One could improve the OB that way. In the off-chance that this is insensitive, it could be an
excellent propellant powder.
[Edited on 5-12-2014 by deltaH]
|
|
|
Dany
Hazard to Others
  
Posts: 482
Registered: 3-8-2013
Member Is Offline
Mood: No Mood
|
|
deltaH,
After doing some theoretical calculations your molecule do not offer any improving over existing structure in term of crystal density and detonation
performance.
The theoretical density of your molecule is ρ= 1.707 g/cm3
This density was calculated using the group additivity method of SHREEVE et al. [1] which take into account correction for second order effects
(presence of hydrogen bonding).
Two simple methods described by KESHAVARZ [2,3] for the calculation of detonation performance (D & P) were used here.
For your structure: D= 8.56 km/s, P= 327 kbar.
with these data in hand it is clear that your molecule has performance lower than that of RDX.
for RDX the experimental density and detonation performance are taken from ref. [4].
ρ= 1.80 g/cm3
D= 8.75 km/s, P= 347 kbar.
Finally, you are saying that your structure will possess excellent detonation performance and insensitivity...are you sure? to me it is just the
opposite...putting nine nitrate ester in this structure will boost sensitivity. If one will attempt the synthesis of this compound he should be very
careful because it is very likely that this structure will be sensitive.
References:
[1] C. Ye, J. n. M. Shreeve, Journal of Chemical & Engineering Data 2008, 53, 520-524.
[2] M. H. Keshavarz, J. Hazard. Mater. 2009, 166, 762-769.
[3] M. H. Keshavarz, J. Hazard. Mater. 2009, 166, 1296-1301.
[4] Charles L. Mader, Numerical Modeling of Explosives and Propellants, Third Edition, 2008.
Dany.
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Thank you so much Dany, I appreciate your calculations greatly. I am a little disappointed with the density, was hoping for at least
1.8. What about the dodecanitro version?
In light of the additional nitro groups suggested by PHILOU Zrealone, I also think it is likely to be sensitive. Do not worry, I am
not planning to prepare it, but it's good to have this warning up anyhow.
Heuristically speaking, when energetic molecules get bigger, do they become more sensitive? From petroleum theory, I know that longer linear alkanes
have lower octane numbers, while branched ones have higher. Perhaps this analogy is extendable to energetics, making dendrimers more insensitive?
Anyhow, just some thoughts that occur to me.
[Edited on 5-12-2014 by deltaH]
|
|
|
Dany
Hazard to Others
  
Posts: 482
Registered: 3-8-2013
Member Is Offline
Mood: No Mood
|
|
deltaH,
Sensitivity of energetic compounds is a very complex topic because for a given compound the sensitivity depend both on chemical and physical
properties. It is not a question of "bigger" or "smaller" what determine the sensitivity. For example, nitromethane and methyl nitrate are both
"small" molecule compared to TNT, HMX, etc...However, nitromethane is so insensitve and require (in the absence of a sensitizers like amine) a large
booster explosive to induce detonation. On the other hand, methyl nitrate which is comparable in size to nitromethane is sensitive. In fact, the
nature of the explosophoric group will determine here the overall sensitivity of the compound. Nitrate ester (e.g., methyl nitrate) as a general rule
are more sensitive than nitroaliphatics like nitromethane.
As I told you, the sensitivity depend both on chemical and physical properties. The geometry (cylindrical, spherical, wedge, etc..) of the tested
explosive can have big influence on sensitivity.
For some explosive sensitivity depend on the orientation of the shock wave inside the crystal of the explosive. We call this shock anisotropy. PETN
crystals shows different sensitivity which depend on the orientation of the shock wave inside the crystal lattice.
Dany.
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Thanks for the explanation Dany. I had a feeling it wasn't simple... now confirmed by you 
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
With Dany's permission, I thought I'd mention a point he made on my energetic dendrimer 2 thread that has bearing on this discussion.
In the paper (1) referenced by Dany, it was found that for the nitration of the aniline derivative, the melamine amines did not
nitrate with mixed acid alone (that nitrated the aniline groups only), but only with strong nitrating conditions (HNO3+acetic anhydride) did the
nitration proceed further to the melamine amine groups, resulting in a thermally unstable product.
While I appreciate that the alkyl group of TRIS is possibly more activating to the nitrogen than a benzene ring, I am hopeful that employing milder
nitrating conditions can give the nona-nitro product selectively. But again, this is all hypothetical.
(1) M. D. Coburn, J. Heterocycl. Chem. 1966, 3, 365-366.
[Edited on 7-12-2014 by deltaH]
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Quote: Originally posted by deltaH  | Point taken. Thanks for the excellent explanation.
Well then, this becomes quite the beast... I christen it Dendrimite 
Any chance the nitro groups sit on the ring positions with the double bonds radiating outwards?
Anyhow, this thing doesn't look insensitive anymore, but at least it looks like it could pack quite a punch, perhaps similar to PETN?
[Edited on 5-12-2014 by deltaH] |
Appropriate name would much more be like:
2,4,6-tris-(trimethylol-nitraminomethane)-1,3,5-triazine nonanitrate ester (TTMNATANN)
or a more alchemistric name cyanuric-tri-nitroTRIS (CTNT).
But dendrimite or deltaH-ite seems ok 
There is little chance the nitro group switch from the external nitrogen atoms into a triazane (thus related to RDX) ring with loss of aromaticity of
the triazine ring...although it may occure in slight % transistion state and increase sensitivity (unfavorable conformation), or decrease it (extra
resonance form thus an extra way to catch and dissipate energy).
The punch will indeed be in the range of PETN and the sensitivity too, based on related structures(*)...the weakest link is the nitramino moeity...and
not the nitrate ester part.
(*)
The aza atom inside the Cyanuric/triazine ring is very close to the effect of a nitrogroup at the same position...acidity of trinitrophloroglucidol
(1.3.5-trihydroxy-2.4.6-trinitrobenzene) and of cyanuric acid (1.3.5-trihydroxy-2.4.6-triazine) shows this; but also the relative reactivities of
trichlorotrinitrobenzene vs cyanuric trichloride....
Tetryl (CH3-N(NO2)-C6H2(NO2)3)
Pentryl (O2N-O-CH2-CH2-N(NO2)-C6H2(NO2)3)
Heptryl ((O2NOCH2)3C-N(NO2)-C6H2(NO2)3)
PETN (C(CH2ONO2)4)
TMDN (O2NOCH2-CH2-CH2-ONO2)
BDDN (CH3-CHONO2-CH2-CH2ONO2)
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Quote: Originally posted by PHILOU Zrealone  |
Appropriate name would much more be like:
2,4,6-tris-(trimethylol-nitraminomethane)-1,3,5-triazine nonanitrate ester (TTMNATANN)
or a more alchemistric name cyanuric-tri-nitroTRIS (CTNT).
But dendrimite or deltaH-ite seems ok  |
In hindsight, dendrimite is more suited to being a class name for energetic dendrimers in general, rather than for a particular compound 
|
|
|