deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Energetic dendrimers 2: "cyanuric picrates"
Continuing with my theme of energetic dendrimers, I want to propose another. In the review paper attached (1), I noticed that beside for amines,
phenolic derivatives could be condensed with a cyanuric chloride-like derivative to yield not melamine centred dendrimers, but cyanurates. This gave
me the idea of forming a "cyanuric picrate" or perhaps more correctly, a tris(trinitrophenyl) cyanurate:
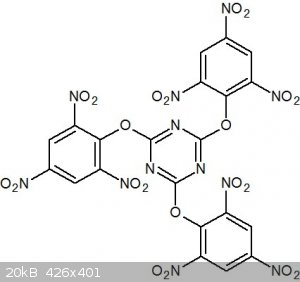
This structure would probably be twisted into a propeller shape to minimise the steric hindrance of the nitro groups.
I thought it might hypothetically be prepared by from reacting three equivalents ammonium picrate and cyanuric chloride in ethanol, forming
ammonium chloride and the cyanuric picrate?
Is the organic chemistry proposed even plausible?
I don't expect this to have particularly special explosive performance, but it might have interesting explosive properties... possibly making it
useful once characterised. As I've stated before, energetic dendrimers may be useful in binary combinations with other energetic materials. I'm also
hoping that maybe this version of an energetic dendrimer has a chance of being insensitive.
Reference (attached for your convenience):
(1) Mackay B. Steffensen, et al. Dendrimers Based on [1,3,5]-Triazines. J Polym Sci Part A: Polym Chem 44: 3411–3433, 2006.
Attachment: Dendrimers Based on [1,3,5]-Triazines.pdf (1.2MB)
This file has been downloaded 620 times
[Edited on 6-12-2014 by deltaH]
|
|
|
Dany
Hazard to Others
  
Posts: 482
Registered: 3-8-2013
Member Is Offline
Mood: No Mood
|
|
deltaH,
In 1966, the known explosive chemist Micheal D. COBURN from the Los Alamos National Laboratory (LANL) synthesized several s-triazine based
energetic materials [1] similar to yours. Two of these structure along with their experimental density are presented below:

Compound (I) can be prepared via two steps: first the reaction of aniline with cyanuric trichloride and then nitrating the
formed product with concentrated HNO3/H2SO4 at 70°C for 3 hours.
Further nitration of (I) with a mixture of acetic anhydride/HNO3 mixture give you compound (II) which is
unstable (rapid decomposition at 100°C and slow decomposition at 0°C).
Picrate salt are weak nucleophile so it is unlikely that a picrate salt will react with cyanuric trichloride to give your proposed structure. A
multi-step synthesis is required. Triaryl Cyanurates can be easily synthesized by the reaction of cyanuric trichloride with sodium salt of hydroxyaryl
compounds (ArONa) in water under microwave irradiation or under other classical conditions (see ref. [2] and references therein). For example, the
sodium salt of para-nitrophenol react with cycanuric trichloride in water under microwave condition to yield the corresponding triaryl
cyanurate (see picture below) in 85% yield. The reaction time was only 1 minute.

This product can be further nitrated to the corresponding 2,4,6-trinitroderivative.
References:
[1] M. D. Coburn, J. Heterocycl. Chem. 1966, 3, 365-366.
[2] A. D. Sagar, D. S. Patil, B. P. Bandgar, Synth. Commun. 2000, 30, 1719-1723.
Dany.
[Edited on 7-12-2014 by Dany]
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
I am very grateful Dany for this EXTREMELY excellent information on the topic! Thank you so much.
My main concern about picrates indeed was one of nucleophilicity. Originally, I had thought if that were the case, cyanuric chloride could be
condensed with phenol followed by nitration. Is there a good/critical reason to use the nitrophenol to derive (III) instead of starting with phenol?
Your structures (I) and (II) have bearing on my first energetic dendrimer thread regarding the compound I called dendrimite, the PETN-like version of
this class of compounds, but with a melamine centre. PHILOU Zrealone had suggested there that the amines of the melamine unit would
nitrate further and in light of this, we suspected sensitivity... turns out it is also likely to be unstable from your evidence of the related
compound (II). However, a very important point that has come out of this is that the nitroamines might be avoided by nitration using milder reagents,
e.g. mixed acids only. This is potentially breakthrough info IMHO.
Also for the sake of synthetic simplicity, I am wondering if phenol, what with its low melting point, cannot be reacted directly with cyanuric
chloride (solvent free) to eliminate HCl with heating.
Thanks again!
[Edited on 7-12-2014 by deltaH]
|
|
|
Dany
Hazard to Others
  
Posts: 482
Registered: 3-8-2013
Member Is Offline
Mood: No Mood
|
|
deltaH,
4-Nitrophenol sodium salt is commercially available
So by using the nitrated phenoxide in the first step you can save time and nitric acid in the second step (the nitration step). However, phenol can
also be used in the first step if the mononitrated derivative is not available.
Dany.
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Good to know, I was merely thinking in terms of cost though... phenol being dirt cheap. Also phenol melts at a mere 40.5C but boils at 181.7C, so was
thinking of the practical advantage to be gained by hypothetically reacting cyanuric chloride and phenol directly, heating to drive off HCl.
[Edited on 7-12-2014 by deltaH]
|
|
|
Dany
Hazard to Others
  
Posts: 482
Registered: 3-8-2013
Member Is Offline
Mood: No Mood
|
|
There is a good and facile way described by Thurston et al. [1] to synthesize triphenyl cyanurate and this by reacting cyanuric trichloride with
phenol in the presence of sodium hydroxide and acetone as solvent. The procedure is as follows:

You can see that the reaction works very well (95% yield) and can be performed on multigram scale. The authors also mentioned that cyanuric
trichloride would not react completely in the absence of organic solvent even at 75°C.
References:
[1] J. T. Thurston, F. C. Schaefer, J. R. Dudley, D. Holm-Hansen, J. Am. Chem. Soc. 1951, 73, 2992-2996.
Dany.
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Wonderful Dany, I appreciate this greatly! Fantastic yields indeed.
Effectively the only remain unknown is that of the nitration of phenyl cyanurate. To me, the key question would be whether simple mixed acids could
affect it?
[Edited on 8-12-2014 by deltaH]
|
|
|
Dany
Hazard to Others
  
Posts: 482
Registered: 3-8-2013
Member Is Offline
Mood: No Mood
|
|
One should try the nitration method used by COBURN for the synthesis of the picrylamino derivative (I).
Dany.
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Yes, I think the stage is now fully set thanks to you. I hope someone with the necessary skill and right to do so reports their results here someday.
|
|
|
Dany
Hazard to Others
  
Posts: 482
Registered: 3-8-2013
Member Is Offline
Mood: No Mood
|
|
deltaH,
A related s-triazine based energetic materials called PL-1, displays important chemical and physical properties. PL-1 was synthesized by
Indian scientist [1] at the High Energy Materials Research Laboratory , Pune, India. The structure of PL-1 along with it's experimental crystal
density is shown below:

As one can see PL-1 possess astonishing crystal density of 2.02 g/cm3 which is one of the highest reported density for an energetic
material.
The synthesis of PL-1 is achieved via 3 steps:
1) reaction of 3,5-dichoroaniline with cyanuric trichloride 2) nitration of the resulting product 3) reaction with ammonia which displays the chlorine
atoms and form PL-1. However, the reported overall yield is relatively low (31%).

The authors estimated the detonation velocity of PL-1 by the method described by Rothstein & Petersen [2]. The method is simple and require only
the chemical composition and the density of the explosive. Based on d= 2.02 g/cm3 the calculated detonation velocity is 7861 m/s. The
detonation pressure is 312 kbar. Detonation pressure can be obtained by the following formula:
P= ρD2/ɣ+1, where D is the detonation velocity (in km/s), ρ is the density, ɣ is the adiabatic coefficient of the detonation gases at
the Chapman-Jouguet point (as an approximation ɣ= 3).
However, a more recent theoretical study [3] (done using DFT computational method) on PL-1 reveals that this molecule has a heat of formation of
ΔHfO= +427.6 kJ/mol. Based on this heat of formation and the reported crystal density of 2.02 g/cm3 the authors estimated the
detonation velocity and pressure by the Kamlet-Jacobs [4] method which is better than Rothstein & Petersen method cited above.
The calculated D and P are: D= 8500 m/s; P= 355 kbar. Detonation performance close to RDX.
Finally it should be noted that PL-1 is a thermally stable and insensitive explosive. PL-1 display a DTA (Differential Thermal Analysis) exotherm at
335°C.
References:
[1] V. K. Bapat, A. K. Sikder, Mehilal, B. G. Polke, J. P. Agrawal, J. Energ. Mater. 2000, 18, 299-309.
[2] L. R. Rothstein, R. Petersen, Propellants, Explos., Pyrotech.1979, 4, 56-60.
[3] X.-H. Ju, Z.-Y. Wang, J. Energ. Mater. 2008, 27, 51-62.
[4] M. J. Kamlet and S. J. Jacobs, J. Chem. Phys. 48 (1), 23–35 (1968).
Dany.
[Edited on 8-12-2014 by Dany]
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
I did my final year research project in Pune at the National Chemical Laboratory on an exchange program 
PL-1 is impressive and clever chemical design/synthetic methodology.
Thanks again for the valuable literature contribution!
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Please be cautious !
Ar-NH2 and Ar-NH-Ar do form upon nitration Ar-NH-NO2 and Ar-N(NO2)-Ar.
The nitraniline compound is unstable and free NO2(+) to another place on the ring in exchange of a H(+).
So yes Ar-N(NO2)-Ar is "unstable" vs Ar-N(NO2)-Alk especially if both Ar ring are strong electro-withdrawing groups like triazine or picryl.
So what is true for Ar-NH-Ar might not be true for Ar-NH-Alk like the TRIS variant you mentionned in another tread.
--> Tetryl and Heptryl are known existing molecule with practical explosive applications.
Thanks Dany,
the PL-1 molecule is one more proof that polymeric material are a good way to increase density, power and VOD and decrease sensitivity. Do you have by
chance the datas for the same molecule as PL-1 but with a TATNB (triaminotrinitrobenzene) core instead of a melamine (triamino-triazine) one?
Note that the final stage with NH3 in the synthesis of PL-1 could be done with CH3-NH2 to form after nitration a tetryl related PL-1.
Also note that PL-1 could react in dendrimer extension reaction with trinitrochlorobenzen to add an extra perimeter of 6 picryl on the 6 external
amino groups.
The resulting planar structure should display even more interesting properties (d, IS, VOD, Energy of formation and detonation)
PL-1 may react further with cyanuric tri-chloride to extand the molecule to a 2D polymer (or 3D polymer) alterning triazine and TNB ring bridged by
-NH- groups...
PL-1 should be acidic by the NH inner core and external NH2...so it might form energetic salts of interesting properties, like by order of
increasingly interesting properties:
-hydrazinium
-hydroxylamonium
-H2N-CH2-C(NO2)3 (trinitroethylamonium)
-H2N-NH-CH2-C(NO2)3 (trinitroethylhydrazinium)
[Edited on 9-12-2014 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Dany
Hazard to Others
  
Posts: 482
Registered: 3-8-2013
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by PHILOU Zrealone  | Please be cautious !
Ar-NH2 and Ar-NH-Ar do form upon nitration Ar-NH-NO2 and Ar-N(NO2)-Ar.
The nitraniline compound is unstable and free NO2(+) to another place on the ring in exchange of a H(+).
So yes Ar-N(NO2)-Ar is "unstable" vs Ar-N(NO2)-Alk especially if both Ar ring are strong electro-withdrawing groups like triazine or picryl.
So what is true for Ar-NH-Ar might not be true for Ar-NH-Alk like the TRIS variant you mentionned in another tread.
--> Tetryl and Heptryl are known existing molecule with practical explosive applications.
Thanks Dany,
the PL-1 molecule is one more proof that polymeric material are a good way to increase density, power and VOD and decrease sensitivity. Do you have by
chance the datas for the same molecule as PL-1 but with a TATNB (triaminotrinitrobenzene) core instead of a melamine (triamino-triazine) one?
Note that the final stage with NH3 in the synthesis of PL-1 could be done with CH3-NH2 to form after nitration a tetryl related PL-1.
Also note that PL-1 could react in dendrimer extension reaction with trinitrochlorobenzen to add an extra perimeter of 6 picryl on the 6 external
amino groups.
The resulting planar structure should display even more interesting properties (d, IS, VOD, Energy of formation and detonation)
PL-1 should be acidic by the NH inner core and external NH2...so it might form energetic salts of interesting properties, like by order of
increasingly interesting properties:
-hydrazinium
-hydroxylamonium
-H2N-CH2-C(NO2)3 (trinitroethylamonium)
-H2N-NH-CH2-C(NO2)3 (trinitroethylhydrazinium)
[Edited on 9-12-2014 by PHILOU Zrealone] |
Philou,
My search didn't yield and results for the TATNB core instead of a melamine. The high density of PL-1 is NOT the result of the polymerisation but by
the presence of multitude of intra- and intermolecular hydrogen bonding between the amino and the nitro groups. If the amino groups were replaced by
methyl the density will be far less than 2.02 g/cm<sup>3</sup>.
To design a dense explosive one should think about dense molecule that yield good crystal packing. Polycyclic molecule are known to be good
candidate. Example include TEX (4,10-Dinitro-2,6,8,12-tetraoxadodecane) an explosive [1] with very high density (d= 1.985
g/cm<sup>3</sup> . .
References:
[1] E.-C. Koch, Propellants, Explosives, Pyrotechnics 2014, DOI: 10.1002/prep.201400195
Dany.
[Edited on 9-12-2014 by Dany]
|
|
|
Dany
Hazard to Others
  
Posts: 482
Registered: 3-8-2013
Member Is Offline
Mood: No Mood
|
|
Until now the discussion is focused on the derivatives of 1,3,5-Triazine as energetic materials. However, it should be kept in mind that
1,3,5-Triazine possess two other positional isomers: 1,2,3-Triazine and 1,2,4-Triazine shown below:

A theoretical study [1] was performed on all possible nitro derivatives of the three rings using DFT and ab initio computational methods. The
fully nitrated structures are shown below:

As one can notice, structure (III) has the highest calculated crystal density (1.93g/cm<sup>3</sup> while structure (II) has the highest Heat Of Formation (HOF) and also the highest detonation performance (calculated
using the Kamlet-Jacobs method). while structure (II) has the highest Heat Of Formation (HOF) and also the highest detonation performance (calculated
using the Kamlet-Jacobs method).
References:
[1] YAKUP ÇAMUR, A COMPUTATIONAL STUDY ON NITROTRIAZINE DERIVATIVES, MIDDLE EAST TECHNICAL UNIVERSITY, 2008, Master thesis.
[2] M. J. Kamlet and S. J. Jacobs, J. Chem. Phys. 48 (1), 23–35 (1968).
Dany.
[Edited on 9-12-2014 by Dany]
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Wow, these calculated heats of formation are extremely largely positive, which makes me doubt them, strongly. For comparison experimental dHf for TNT
= -63.2 kJ/mol, picric acid = -217.9 kJ/mol and RDX = 66.5kJ/mol.
I've always been taught that the heats of formation calculated by semiempirical methods, like PM3 the authors used AFAIK, are in fact seldom reliable.
In my personal experience, I could only get reliable dHf (gas) values by using high level quantum mechanical models if I used the same basis sets and
also calculated the heats of formation of all the elements involved in their standard states (including a computationally expensive periodic
calculation for graphite) and then subtracted that from the product, which if solids were involved, mandated the use of a periodic calculation.
For hypothetical solids, this is very difficult as you need to know/predict the crystal structure and the computation becomes very resource intensive.
[Edited on 10-12-2014 by deltaH]
|
|
|
Dany
Hazard to Others
  
Posts: 482
Registered: 3-8-2013
Member Is Offline
Mood: No Mood
|
|
deltaH,
The semi-empirical method PM3 is sufficiently accurate to calculate the heat of formation of energetic compounds. Keshavarz et al. founds in a a
recent study [1] that the condensed heat of formation of nitroaromatic compounds can be accurately calculated via the PM3 method. however, I
would like to add something. When calculating detonation performance of energetic materials, obtaining very accurate HOF values is not a big concern
for us. On the other hand, density should be very accurately predicted since detonation performance (Detonation velocity and pressure) are very
sensitive to density variation. Detonation velocity in proportional to density while detonation pressure is proportional to density squared.
References:
[1] M. OFTADEH1, M. H. KESHAVARZ and R. KHODADADI, Prediction of the Condensed Phase Enthalpy of Formation of Nitroaromatic Compounds Using the
Estimated Gas Phase Enthalpies of Formation by the PM3 and B3LYP Methods, CEJEM, 2014, 11(1), 143-156.
Dany.
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
So one needs only the gas phase heats of formation, not the solid? I didn't realise that, my bad.
Density from a molecular modelling point of view is hard to accurately predict for hypothetical EM structures... I've tried it several times, but
honestly, it still seemed to me like a very woolly science (the density predictions, not molecular modelling per say). Eventually I decided it wasn't
worth the effort (or computational wait for that matter  ) )
[Edited on 10-12-2014 by deltaH]
|
|
|
Dany
Hazard to Others
  
Posts: 482
Registered: 3-8-2013
Member Is Offline
Mood: No Mood
|
|
Generally, gas phase heat of formation tend to overestimate the calculated detonation performance although in the past detonation velocity has been
successfully calculated using the gas phase HOF [1]. Our experience shows that condensed crystal density can be accurately predicted using
computational (DFT) or group additivity methods. Of course, the presence of second order effects (e.g., hydrogen bonding, polymorphs...) in the
condensed phase tend to introduce deviations in the calculated density.
References:
[1] Keshavarz MH., A simple approach for determining detonation velocity of high explosive at any loading density, J Hazard Mater.
2005 ;121(1-3):31-6.
Dany.
[Edited on 10-12-2014 by Dany]
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
The stability of the triaza-rings is in the order:
1.3.5 > 1.2.4 > 1.2.3
The mutual presence on the aromatic ring of N atoms and NO2 groups decreases the stability of the later that are very prompt to nitro-nitrite
rearrangement and subsequent hydrolysis...
Ar-NO2 <==> Ar-O-N=O
Ar-O-N=O + H2O ==> Ar-OH + HO-N=O
This, and the high positive heat of formation explains the probable inexistance of those compounds and to date the unsucessful isolation of
sym-trinitrotriazine.
[Edited on 10-12-2014 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Thanks Dany for you patient mentorship. Yes and is exactly these second order effects that can make the important difference or so it
seems.
PHILOU, good point too, thanks.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Dany,
There must be something else...for PL-1 to reach 2.02 g/ccm
1)Both free "monomeric" molecules display lower densities than PL-1 while diplaying the same atom-groups and thus the same subsequent NH2-NO2 and
NH2-N interractions...
a)1.3.5-Triamino-2.4.6-trinitrobenzene (TATB) density: 1.93g/ccm
b)2.4.6-Triamino-1.3.5-triazine (cyanuric triamide/melamine) density: 1.574 g/ccm
c)a mix of 3/4 TATB and 1/4 melamine would have a density of approx 1.84 g/ccm
2)The free molecules do have more freedom of move than the arms of PL-1 vs its core to ensure best packing for cristal lattice. Also PL-1 should
display structural voids.
--> I really think it has to do with the condensation/polymerisation patern.
For obvious reasons CH3 is a bad densophore group vs NH2 and NO2, so your example is a bit tricked  . .
PL-1 is a polycyclic compound and so it enters your rule...just as my putative 2D or 3D polymer  . .
TEX is a caged polycyclic compound.
I repeat here that finite molecule are a limitation for the density increase purpose!
As an image, a simple study of the density of dry sand, silice glass and silice cristal (all 3 SiO2)should give you a hint:
-Dry sand (representing finite molecules packing) d=1.6
-Silice glass (representing 3D polymeric) d=2.53
-Silice cristal (representing 3D max cristallinity polymer) d=2.65
Where does this come from? This is due to the contraction of volume resulting from the creation of chemical bonds...those are by definition way
shorter than H bondings molecular interactions ...the only interaction comming close to chemical bonding is ionic charge interactions (like in
salts...and this explains that CHNO salts display good densities).
Quote: Originally posted by Dany  | Quote: Originally posted by PHILOU Zrealone  |
Thanks Dany,
the PL-1 molecule is one more proof that polymeric material are a good way to increase density, power and VOD and decrease sensitivity. Do you have by
chance the datas for the same molecule as PL-1 but with a TATNB (triaminotrinitrobenzene) core instead of a melamine (triamino-triazine) one?
Also note that PL-1 could react in dendrimer extension reaction with trinitrochlorobenzen to add an extra perimeter of 6 picryl on the 6 external
amino groups.
The resulting planar structure should display even more interesting properties (d, IS, VOD, Energy of formation and detonation)
PL-1 may react further with cyanuric tri-chloride to extand the molecule to a 2D polymer (or 3D polymer) alterning triazine and TNB ring bridged by
-NH- groups...
|
The high density of PL-1 is NOT the result of the polymerisation but by the presence of multitude of intra- and intermolecular hydrogen bonding
between the amino and the nitro groups. If the amino groups were replaced by methyl the density will be far less than 2.02
g/cm<sup>3</sup>.
To design a dense explosive one should think about dense molecule that yield good crystal packing. Polycyclic molecule are known to be good
candidate. Example include TEX (4,10-Dinitro-2,6,8,12-tetraoxadodecane) an explosive [1] with very high density (d= 1.985
g/cm<sup>3</sup> . .
|
[Edited on 11-12-2014 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
Hydrogen bonding can even lower density for solids, here's why:
Consider the richly hydrogen-bonded melamine cyanuric acid 1:1 complex, the most extensively/densely hydrogen bonded material I know of, off the top
of my head. It has a Specific Gravity of only 1.7.Compare to the SG of pure cyanuric acid (2.5) and melamine (1,6), so the hydrogen bonding actually
made the density less than what you would expect (something closer to 2.05).
If you look at the structure of the complex, you see that by alligning all the hydrogen bonds, one forces the solid structure into a packing that has
more wasted space.
*****************
I agree with PHILOU, this is about bond lengths. In a way, dendrimers can be thought of as a 2d compromise between the 3d bonding of
caged energetics and 1d discretes. The compromise on density is also one of difficulty of synthesis.
As such, I believe they may hold valuable promise 
[Edited on 11-12-2014 by deltaH]
|
|
|
Dany
Hazard to Others
  
Posts: 482
Registered: 3-8-2013
Member Is Offline
Mood: No Mood
|
|
A long discussion between me and the great PHILOU Zrealone about density increase in energetic materials exist here:
http://www.sciencemadness.org/talk/viewthread.php?tid=29350#...
I'm still waiting references that promote polymerization as an effective way for density increase in energetic materials.
Dany.
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
I won't get involved in that train of thought too much. Strictly speaking, I'm advocating oligomerisation for density increase, not polymerisation per
say.
Oligomeric materials, like these simple dendrimers, are highly crystalline and of fixed molecular weights where polymers are not, which gives them a
discerned advantage over the latter. This is yet another reason why energetic dendrimers hold promise in my books.
|
|
|