| Pages:
1
..
3
4
5 |
Crayfi
Harmless

Posts: 2
Registered: 28-2-2015
Member Is Offline
Mood: No Mood
|
|
I know this article is old af but I've been doing a bit of research and believe it may be possible to purify this "red goop" everybody has extracted
from their peppers.
You see, the red color comes from a high concentration of beta carotene. The beta carotene and sugars everybody has inevitably extracted along with
the capsaicinoids is probably imparting a bit of flavor as well.
So here's the theory: Beta-carotene isn't soluble in glycerin. Sugars aren't usually soluble in ether. Ether isn't miscible with glycerin. (see where
this is going?)
My proposal is to mix the red goop with some of the usp glycerin you can get at the pharmacy (I get mine from Walmart). It's a fairly viscous liquid
so some heating may be required for adequate mixing then let it cool to room temp to separate into layers. Pour off the glycerin and mix it with some
food grade ether (Purify sulfuric acid with activated charcoal then mix with ethanol and distill to give ether. Fractionally distill again to remove
any ethanol and drop on a watch glass to ensure complete evaporation). The ether and glycerin will form two separate layers but the glycerin may
require a little preheating for good mixing. Evaporate off the ether and now the red goop is a mix of capsaicinoids from the peppers you mutilated.
EDIT: Not sure on the viability of using glycerin to separate out the beta-carotene. Heated some glycerin and put in with my red goop and everything
is now dissolved... Maybe it has to do with having sugars in the mix. Whatever it is it's no bueno. More research revealed the citation for
beta-carotene insolubility with glycerin on Wiki is no longer valid returning the all-too-familiar response of page not found on the host website.
There's still hope.
Uses hexane but it will only dissolve ~20mg/L with a Lutein and beta-Carotene mix. http://pubs.acs.org/doi/pdf/10.1021/jf00015a013
Capsaicin is routinely extracted with hexanes. http://www.cals.ncsu.edu/specialty_crops/publications/report...
[Edited on 13-8-2015 by Crayfi]
|
|
|
Crayfi
Harmless

Posts: 2
Registered: 28-2-2015
Member Is Offline
Mood: No Mood
|
|
Update: The glycerin method for separating capsaicinoids and beta carotene works. First I took my glycerin/pepper extract mix and added some fresh
ether. No emulsion was formed during shaking. Put the mix in the freezer for around an hour and a half to let the glycerin thicken. The ether layer is
the top layer, this was poured off and evaporated. What was left in the watch glass was beta carotene and a mix of glycerin and capsaicinoids. Not
sure if it was the sugar in the original mix that caused the glycerin to mix in with it (I think it was the sugars) but with an ether extract this is
no longer a problem.
Again, make sure your ether evaporates with no residue left over and make sure to add baking soda or sodium carbonate to be sure it isn't acidic if
produced by ethanol+sulfuric acid method. If these two conditions aren't met I would really suggest not eating the product.
|
|
|
Eddygp
National Hazard
   
Posts: 858
Registered: 31-3-2012
Location: University of York, UK
Member Is Offline
Mood: Organometallic
|
|
If DMSO can readily dissolve the stuff, it would even be possible to use it as a chemical weapon. Just to realise how potent capsaicin is... the very
low concentrations at which humans can detect -and, probably, suffer- it. DMSO is extremely easy to absorb through the skin, which means that a
certain concentration of capsaicin in solution would be absorbed, with all of the negative effects that it would mean. Pure capsaicin can cause
blisters. If you absorb it, I don't even want to imagine what can happen.
Just in case, I'd rather not dissolve any potentially lethal compounds in solvents such as DMSO, because even a brief exposure means that a fairly
significant quantity of the compound has entered your body.
Of course, if you have the appropriate clothing and equipment, it should not be a problem. Safety, safety, safety.
there may be bugs in gfind
[ˌɛdidʒiˈpiː] IPA pronunciation for my Username |
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Nuclear Chili
Overrated
www.sciencemadness.org/talk/viewthread.php?tid=5329&page...
This chili will stop an elephant
www.youtube.com/watch?v=9k-SBpElcWA&t=3m33s
Give one of these to a mean child and tell them it's a strawberry.
http://store.puckerbuttpeppercompany.com/collections/carolin...
Techniques to Disrupt, Deviate and Seize Control of
an Internet Forum In case you wonder W T F ! is going on here
?
www.zerohedge.com/contributed/2012-10-28/cointelpro-techniques-dilution-misdirection-and-control-internet-forum https://web.archive.org/web/20120814124000/www.washingtonsblog.com/2012/08/the-15-rules-of-internet-disinformation.html
___________________________________________________________________________________________________________________
___________________________________________________________________________________________________________________
___________________________________________________________________________________________________________________
|
|
|
Tsjerk
International Hazard
    
Posts: 3022
Registered: 20-4-2005
Location: Netherlands
Member Is Online
Mood: Mood
|
|
I have a but load of Carolina Reaper and Trindad Scorpion peppers, I experimented a bit with extracting the capsaicinoids and found that methanol
extraction off the dried peppers gives a crystalline product. I just magnetically stir 10 grams of dried pepper in 75 ml methanol for 24 hours, filter
the solids of, evaporated the methanol to air and freeze the still liquid extract. There is a white layer of crystals on the bottom of the beaker
glass now.
The mixture is brought to room temperature and 75 ml of water is added and the product is filtered. There is 0.6 gram of solids in the filter paper
with some white crystals in there, but the product is still bright red.
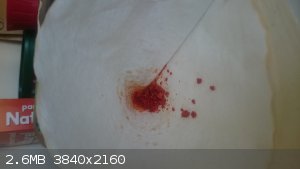
Does anyone have a suggestion for further purification of this? I would love to have some colorless crystals.

Two fresh Trinidads
My plants are doing great, I think I will have hundreds of peppers in the next three months. I think the "dried" peppers I extracted last time were
not really dry, next time I will extract 10 grams of bone dry Carolina reaper. Also grinding them a bit better will probably help.

Edit: I just remembered I had some dried Carolina Reapers somewhere, and I had exactly 10 grams of bone dry peppers. I put them on a magnetic stirrer
with 75 ml methanol. I will filter in two days.
[Edited on 8-6-2019 by Tsjerk]
[Edited on 8-6-2019 by Tsjerk]
|
|
|
Ubya
International Hazard
    
Posts: 1232
Registered: 23-11-2017
Location: Rome-Italy
Member Is Offline
Mood: I'm a maddo scientisto!!!
|
|
Last year I grew 2 plants of Carolina reaper just for this purpose, but I could only get 30-40 peppers, so this year I'm giving another go.
My plan is to dry the peppers in an oven at low heat and then to remove the last bits of moisture with Silica gel kitty litter (it worked) and then to
grind them to a fine powder. Next I would try with a soxhlet extractor using acetone or ethanol as solvent, this way I can extract more pepper mass
with less solvent and in less time. Then by evaporating the solvent I would get the usual red oil/goo every one gets. To remove the colored carotenes
from the capsicinoids I thought of using: a gravity chromatography column using ground Silica kitty litter as stationary phase and petroleum ether as
eluent, or maybe an acid base extraction, but apart from a few mentionings, I couldn't find any real experiences of this method
---------------------------------------------------------------------
feel free to correct my grammar, or any mistakes i make
---------------------------------------------------------------------
|
|
|
Tsjerk
International Hazard
    
Posts: 3022
Registered: 20-4-2005
Location: Netherlands
Member Is Online
Mood: Mood
|
|
Quote: Originally posted by Ubya  | Last year I grew 2 plants of Carolina reaper just for this purpose, but I could only get 30-40 peppers, so this year I'm giving another go.
|
They love tons of sun and water, mine are behind a window on the south, in the photo I just moved the plants from the left to the right and visa
versa, the ones that were on the left got more sun at the end of the day, that is why there is such a difference in size (besides the size of the
pot).
Acetone is a bit crude in my experience, alcohols give a cleaner extract.
Edit: I'm moving the Caroline Reaper in the small pot to a 50 liter pot outside today, it will be in the sun from 11 till sunset, lets see what
happens.
[Edited on 9-6-2019 by Tsjerk]
|
|
|
fusso
International Hazard
    
Posts: 1922
Registered: 23-6-2017
Location: 4 ∥ universes ahead of you
Member Is Offline
|
|
Does acetone react with the extracts?
|
|
|
Tsjerk
International Hazard
    
Posts: 3022
Registered: 20-4-2005
Location: Netherlands
Member Is Online
Mood: Mood
|
|
No, but it extracts about anything except the cellulose, alcohols are more selective.
|
|
|
Ubya
International Hazard
    
Posts: 1232
Registered: 23-11-2017
Location: Rome-Italy
Member Is Offline
Mood: I'm a maddo scientisto!!!
|
|
thank you for the info
---------------------------------------------------------------------
feel free to correct my grammar, or any mistakes i make
---------------------------------------------------------------------
|
|
|
Tsjerk
International Hazard
    
Posts: 3022
Registered: 20-4-2005
Location: Netherlands
Member Is Online
Mood: Mood
|
|
Water messes things up though, I even (thought I) saw a difference between 96% ethanol and 99.9%. so maybe methanol or isopropanol are better solvents
if you don't have anhydrous ethanol.
|
|
|
lordcookies24
Hazard to Self
 
Posts: 78
Registered: 2-1-2019
Location: pluto
Member Is Offline
Mood: curious
|
|
Don’t those chemicals associated with spiciness work by activating the heat sensors in the mouth?
|
|
|
Tsjerk
International Hazard
    
Posts: 3022
Registered: 20-4-2005
Location: Netherlands
Member Is Online
Mood: Mood
|
|
Yep, url
|
|
|
Pumukli
National Hazard
   
Posts: 686
Registered: 2-3-2014
Location: EU
Member Is Offline
Mood: No Mood
|
|
Maybe it would be worth trying extraction first with something apolar, say heptane, petrol ether, something like that. This extraction would remove
fats, waxes, perhaps the coloring carotenoids as well.
Then in the second step extract with something more polar, say acetone or ethyl-acetate.
I'd avoid highly polar solvents, and as were told, water just messes things up. I think even methanol is too polar and might dissolve/extract e.g.
sugars or other polar compounds.
|
|
|
Tsjerk
International Hazard
    
Posts: 3022
Registered: 20-4-2005
Location: Netherlands
Member Is Online
Mood: Mood
|
|
[Edited on 10-6-2019 by Tsjerk]
|
|
|
Tsjerk
International Hazard
    
Posts: 3022
Registered: 20-4-2005
Location: Netherlands
Member Is Online
Mood: Mood
|
|
Quote: Originally posted by Pumukli  | Maybe it would be worth trying extraction first with something apolar, say heptane, petrol ether, something like that. This extraction would remove
fats, waxes, perhaps the coloring carotenoids as well.
Then in the second step extract with something more polar, say acetone or ethyl-acetate.
I'd avoid highly polar solvents, and as were told, water just messes things up. I think even methanol is too polar and might dissolve/extract e.g.
sugars or other polar compounds. |
I have n-hexane... I could give it a go..
|
|
|
Tsjerk
International Hazard
    
Posts: 3022
Registered: 20-4-2005
Location: Netherlands
Member Is Online
Mood: Mood
|
|
I might have found a way to purify.. I extracted 10 grams of dry Carolina Reaper with 75 ml of methanol. Apparently the peppers soaked up 25 ml, but I
rinsed them with another 25 ml. When I put the extract in a cabinet with some CaCl2 to dry (as it also binds methanol and ethanol) I already get
crystals after evaporating 15 ml. I will filter when I get down to about 25 ml.
I'm doing about 15 ml of methanol in 20 hours, not too bad.
Edit: Dissolving the crude capsaicine in methanol and diluting that with water to 66% methanol seems to do the trick. The red stuff becomes filterable
and leaves a white milk like filtrate. I put the filtrate over the CaCl2 (as in a exsiccator) like the methanol extract. I guess that will leave a
white crystalline substance when dry.
[Edited on 11-6-2019 by Tsjerk]
|
|
|
| Pages:
1
..
3
4
5 |