Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
Errors in textbooks
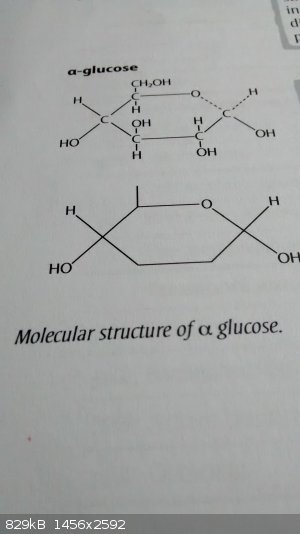
I was perusing a textbook issued by my school earlier today and came across the following figure, which I believe is incorrect. Can someone please
verify that this is the case? It appears that, when drawing the skeletal structure of the pyranose ring (α-glucose), they have omitted 3 hydroxyl
groups which are displayed in the full structure given immediately above.
This may be an error, or a case of over-simplification.
This thread could also be used to discuss other potential errors members have found in the literature.
[Edited on 9-12-2014 by Hexavalent]
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
Artemus Gordon
Hazard to Others
  
Posts: 178
Registered: 1-8-2013
Member Is Offline
Mood: No Mood
|
|
I'm still very weak on O-Chem, but based on the Wikipedia entry for glucose, the only difference I see between the alpha and beta forms is that the
H/OH pair next to the oxygen in the ring is in the same orientation as the pair in the para position in the alpha form, and inverted in the beta form.
Since they already gave you the full diagram, I think the simplified diagram is intended to focus on only those two areas. So I think the diagram is
correct as a teaching tool in this instance.
BTW, how do you enter Greek letters?
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
It was a thought that the Simplified form may have been given in some context that made the other groups irrelevant.
I suspect many other 'errors' might be encountered, if the Context is not seen.
E.g. EtOH doesn't exactly describe ethanol, CH3CH2OH, and so is EtOH an error ?
|
|
|
Zyklon-A
International Hazard
    
Posts: 1547
Registered: 26-11-2013
Member Is Offline
Mood: Fluorine radical
|
|
ALT codes: 224=α, 225=ß, 226=Γ ... etc.
Supposedly, you can use Ctrl+G then type the Latin letter a=α, b=ß... etc. This doesn't work on the computer I'm on now however, but the ALT
codes always work.
|
|
|
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|
| Quote: |
The sodium concentration is closely anti-correlated with isotopic temperature (r**2 = -0.70 over the past 420 kyr).
|
"The correlation is imaginary!"
Petit, J. R., Raynaud, D., Basile, I., Chappellaz, J., Ritz, C., Delmotte, M., … Pe, L. (1999). Climate and atmospheric history of the past 420,000
years from the Vostok ice core , Antarctica. Nature, 399, 429–436.
al-khemie is not a terrorist organization
"Chemicals, chemicals... I need chemicals!" - George Hayduke
"Wubbalubba dub-dub!" - Rick Sanchez
|
|
|
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
Et is used as a pseudoelement symbol for the ethyl group, whereas the OH shows that the compound is an alcohol. Hence, writing EtOH
explicitly means "ethyl alcohol".
The diagram is given as a "side note" in a discussion on the condensation reactions of glucose to form 1,4-glycosidic bonds. Although I appreciate
that substituents may be taken out to improve clarity (as the ones removed do not participate in covalent bonding), it is wrong to remove them without
clarifying this, especially when it is still labelled as α-glucose. By removing those hydroxyl groups, the compound is no longer α-glucose, and so
it should not be labelled as such.
It is important to simplify matters when possible, but not to the extent that a factually incorrect statement is made.
[Edited on 9-12-2014 by Hexavalent]
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
Etaoin Shrdlu
National Hazard
   
Posts: 724
Registered: 25-12-2013
Location: Wisconsin
Member Is Offline
Mood: Insufferable
|
|
That looks horrible. I mean, it could be argued they were trying to show the carbon skeleton, but they left half the hydroxyl groups in.
|
|
|
Chemosynthesis
International Hazard
    
Posts: 1071
Registered: 26-9-2013
Member Is Offline
Mood: No Mood
|
|
I have seen such notation with DNA and RNA to emphasize the oxygenation status of the 2' carbon. Needless to say, I am not a fan. It appears to be
more of a biology nomenclature attempt.
|
|
|
ScienceHideout
Hazard to Others
  
Posts: 391
Registered: 12-3-2011
Location: In the Source
Member Is Offline
Mood: High Spin
|
|
@Hex
No chemistry book publisher would unintentionally leave out hydroxyl groups on a glucose molecule- they just won't. Thus, it must be intentional. The
question is- is it justifiable?
My thought- yes. Completely. Artemus Gordon is correct. There are two forms of cyclic glucose, pertaining to isomerization on the stereochemistry of
the forms. The alpha and beta forms are dependant on whether the hydroxyl groups are axial or equitorial. In this case, the diagram shows, clearly
labeled, alpha glucose, and it can be seen because both hydroxyl groups are pointing in the same direction.
So it is clear that the organic projection that was made by the publisher was intended to simplify the molecule to where the reader looks at these two
spots on the molecule... the important spots where the chemistry is 
My suggestion is that you 'think like the publisher' before assuming an error was made. Authors are careful to avoid such errors, and if there is one,
it will usually be fixed before the book enters the market.
hey, if you are reading this, I can't U2U, but you are always welcome to send me an email!
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Unlikely that there are Zero errors in published works, yet this is possibly a misunderstanding on the part of the Reader rather than the Publisher.
Context is always vitally important.
The decision to Publish must be difficult if one considers oneself an Authority on a subject.
Peer critique would appear Personal if fundamental disagreements were met.
[Edited on 9-12-2014 by aga]
|
|
|
Hexavalent
International Hazard
    
Posts: 1564
Registered: 29-12-2011
Location: Wales, UK
Member Is Offline
Mood: Pericyclic
|
|
Sorry, I should have clarified that this was in a biology textbook. I appreciate your point, but a note should be added explaining the omissions for
simplification purposes; the current format suggests that this is alpha-glucose, whereas it clearly is not. Another argument is that this was a
publication by the exam board, who state on the syllabus that the structure of glucose must be recalled. If a student was to draw the skeletal form
shown here, they would almost certainly lose marks. I understand that removing functional groups which do not contribute significantly to the
chemistry of the molecule is useful to highlight where the important sites are, but this version should not be labelled directly as alpha-glucose.
Besides, the skeletal form is simplified to begin with, so is not necessary or correct to reduce it further.
Finally, although skeletal forms are not required for biology, they are mandated in the chemistry syllabus. Many biology students are also taking
chemistry, and so apparently showing that it is acceptable to omit oxygen atoms may cause confusion elsewhere.
"Success is going from failure to failure without loss of enthusiasm." Winston Churchill
|
|
|
Chemosynthesis
International Hazard
    
Posts: 1071
Registered: 26-9-2013
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Hexavalent  | | Sorry, I should have clarified that this was in a biology textbook. I appreciate your point, but a note should be added explaining the omissions for
simplification purposes; the current format suggests that this is alpha-glucose, whereas it clearly is not. Another argument is that this was a
publication by the exam board, who state on the syllabus that the structure of glucose must be recalled. If a student was to draw the skeletal form
shown here, they would almost certainly lose marks. |
I thought this was from a biology text. I remember being very upset when seeing this type of thing because students definitely get points deducted for
drawing such structures in chemistry, and there are plenty if biologists and professors who know their organic chemistry very well. It's a shame no
consensus appears to be being used. I know when I was in school, I wasn't allowed to mix shorthand in chemistry (carbons as sticks/lines need to be
consistently sticks/lines in a structure).
I also think it is very important to note the dates of text publication. What is known to be wrong now might have been consensus earlier.
[Edited on 9-12-2014 by Chemosynthesis]
|
|
|
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|
When you see it...

al-khemie is not a terrorist organization
"Chemicals, chemicals... I need chemicals!" - George Hayduke
"Wubbalubba dub-dub!" - Rick Sanchez
|
|
|
j_sum1
Administrator
       
Posts: 6221
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
Nah. I missed it. You will have to show me.
All I got was that it projects into the future, but I assume the physical data will remain the same in 2016.
|
|
|
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|
The formula is wrong in the One Before 909; citric acid is not quadraprotic!
You do raise a good question, though: what properties are safe to project into the future, and which aren't? Philosophers of science are still trying
to figure that one out...
https://en.wikipedia.org/wiki/New_riddle_of_induction
al-khemie is not a terrorist organization
"Chemicals, chemicals... I need chemicals!" - George Hayduke
"Wubbalubba dub-dub!" - Rick Sanchez
|
|
|
fluorescence
Hazard to Others
  
Posts: 285
Registered: 11-11-2013
Member Is Offline
Mood: So cold outside
|
|
I think this is not even that wrong but I so loved the way it's drawn that I had to post it here when I saw
that glucose above. Those were structural ideas for certain coordinations. I had to look for a certain article on
thermal reactions of lanthanide-ferricyanides to their corresponding lanthanide-cyanide and needed a
certain Volume of the Russian Journal of Inorganic Chemistry that wasn't even on their main site anymore
and nowhere to be found on the net since it was quite old. Luckily our university had it and so I just skipped
through some chapters till I ended on these two beautiful ideas. I even tried to copy the left one but it always
takes so much time to write it like this otherwise I'd really start writing tetrahedral and planar coordination compounds
like that  . .
https://app.box.com/s/s1p8k5xagk7b2mbvmseh6e6t6a5ivn38
Edit:
Oh here is another one I found in a book on inorganic chemistry. For my research I have to look through so many very old books and sometimes
you just find gems like this:
https://app.box.com/s/on7god7tmg70abcsw8yyb98kfju9d5yp
If I ever publish anything I'll write everything like that just because it looks cool. Back then you didn't even include the reactions in your papers
and today you have to add dozens of measurements and stuff - time for a revival of the old traditions 
[Edited on 1-9-2015 by fluorescence]
|
|
|