Flip
Hazard to Others
  
Posts: 116
Registered: 7-12-2005
Member Is Offline
Mood: No Mood
|
|
Novel synthesis of methyl ketones from terminal alkenes
I have devised a novel reaction scheme for the synthesis of methyl ketones from terminal alkenes. While the procedure is not directly referenced in
the literature, it has been created using textbook reactions with IDENTICAL functional group chemistry. Each step has decent yeild, all products are
major products, and most importantly, these reactions are proven to work and are taught in major college chemistry courses.
What I would like from you guys is some help in developing a base of references and advice that might help me work through some of the expected
yeilds, ratios, and procedural details, with a particular focus on aromatics ; ) I hope to do a write-up here and would love to cite you guys as
contributors.
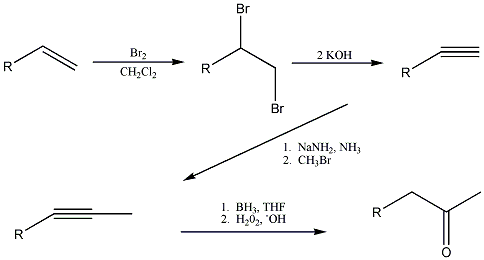
The reaction scheme is in the attached file, but i'll give you rundown... the ketone is formed by the tautomerization of the enol, which is
created by hydroboration of the internal alkyne in tetrahydrofuran. The internal alkyne is formed by the alkylation of the terminal alkyne with
bromomethane. The terminal alkyne is synthesized from the starting alkene by bromination, followed by dehydrohalogenation.
This reaction scheme has potential, especially those of us that migrated here from another board some time ago.
What do you guys think? Can I get some help?
Feel free to PM me on this topic as well.
- Flip
|
|
|
budullewraagh
Hazard to Others
  
Posts: 168
Registered: 1-8-2004
Location: new york
Member Is Offline
Mood: Aliphatic
|
|
KOH and the dibromoalkane would produce the corresponding vicinal diol, not an alkyne. perhaps you were missing an E1 step?
that's an interesting concept with the vicinal en-diol (if that really is a term) causing the removal of water. well done.
|
|
|
chochu3
Hazard to Others
  
Posts: 185
Registered: 21-10-2003
Location: South Side Tejas [Cloverland]
Member Is Offline
Mood: Inside looking out
|
|
It will produce the alkyne after additon of a strong base KOH or NaNH2. The hydroboration would cause a an alcohol on the benzyl carbon. I don't think
you will get a ketone unless you use mercury sulphate on an alkyne or you oxidate the alcohol from the hydroboration, but you will still get a mixture
of products.
\"Abiding in the midst of ignorance, thinking themselves wise and learned, fools go aimlessly hither and thither, like blind led by the blind.\" -
Katha Upanishad
|
|
|
Flip
Hazard to Others
  
Posts: 116
Registered: 7-12-2005
Member Is Offline
Mood: No Mood
|
|
Tautomerization
Quite right about the hydroboration, the initial product will have an -OH on the 2 position of the double bond. This is an enol, which will
tautomerize spontaneously to the ketone. (keto-enol tautomerization)
I thought about mercury sulfate, but that would put the carbonyl on the wrong carbon of the internal alkyne. I've considered HgSO4 for the conversion
of terminal propynes to methyl ketones, however, but to my knowledge mercury sulfate is a watched reagent. Anyone know if there is an easy
preparation for it?
Back to the reaction though, I am certain, in agreement with chochu3, that treatment with a strong base will yeild the alkyne via dehydrohalogenation.
The hydroboration will yeild the enol, which will tautomerize to the ketone.
I don't suppose that anyone has any references that I might use to work up the procedure and stoichiometry for these reactions?
[Edited on 3-3-2006 by Flip]
|
|
|
Flip
Hazard to Others
  
Posts: 116
Registered: 7-12-2005
Member Is Offline
Mood: No Mood
|
|
Regiochemistry of BH3 addition
The hydroboration is an anti-markovnikov addition, and so the BH3, and therefore the OH, should add to the less stable of the two sites where we might
imagine a carbocation. This would be the two position, if we consider the hyperconjugation and resonance from the R group. I believe that it also
makes sense in terms of the steric crowding that could be caused by say, for instance, an aromatic ring.
And in case no one else thought of it, this opens up some interesting new possibilities for styrofoam : )
|
|
|
Flip
Hazard to Others
  
Posts: 116
Registered: 7-12-2005
Member Is Offline
Mood: No Mood
|
|
Triple bond isomerization with KOH
Actually, I would like to tweak the above procedure a little bit.
If the R group is cyclic, this wouldn't matter, but if the R group is an alkyl chain, KOH could actually cause an isomerization of the triple bond to
the next carbon over on the chain (within the R group).
Now the reaction is still solid if the R group isn't a straight chain, but because the dehydrohalogenation can also be accomplished with sodamide, I'm
going to tweak the procedure to utilize the NaNH3 instead of KOH. It's just one less neccessary reagent, the way I see it.
I do believe that I have devised a whole new route to 2-propanones. : )
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
Other than sticking the methyl on the alkyne, this is almost exactly like a question I got on my intoductory o-chem final. It was something like
prepare R-CO-CH3 from R-CH=CH2, obviously in this case oxymercuration was used instead of hydroboration though. So I am afraid its not a completly
new idea, but the addition of the methyl step is very nice in that one would not have to work with mercury compounds.
|
|
|
Flip
Hazard to Others
  
Posts: 116
Registered: 7-12-2005
Member Is Offline
Mood: No Mood
|
|
Of course I didn't invent the reactions, just the synthesis strategy ; ) It's kinda like building with legos, each reaction you learn adds to your
collection of legos until eventually you can build any molecule you want. : )
For the record though, no one to my knowledge in or out of the realm of "amateur experimentalism" has ever synthesized 2-propanones from ethenes
using this strategy... especially the ones that I have in mind. And for all I know, there could be a more efficient strategy that is
preferred by the Phd crowd.
However, giving credit where credit is due, I do have to thank my professor, who judging by his orations is probably also reading this. : )
[Edited on 4-3-2006 by Flip]
|
|
|
Flip
Hazard to Others
  
Posts: 116
Registered: 7-12-2005
Member Is Offline
Mood: No Mood
|
|
Actually I should thank you though rogue... because that's exactly what I need... people who have seen something similar to this before, and who might
be able to cite journal articles that could give me some more insight into the procedural details of each step.
Surely the same reactions on similar or equivalent functional group chemistry are performed and published all over the place... I have searched JACS
but my queries haven't turned up as much as i'd like. Does anyone know any ref's that might help me work out ratios, etc? Would anyone be willing to
collaborate with me on this?
Thanks,
Flip
[Edited on 4-3-2006 by Flip]
|
|
|
Ionium
Harmless

Posts: 6
Registered: 20-6-2005
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Flip
Quite right about the hydroboration, the initial product will have an -OH on the 2 position of the double bond. This is an enol, which will
tautomerize spontaneously to the ketone. (keto-enol tautomerization)
I thought about mercury sulfate, but that would put the carbonyl on the wrong carbon of the internal alkyne. I've considered HgSO4 for the conversion
of terminal propynes to methyl ketones, however, but to my knowledge mercury sulfate is a watched reagent. Anyone know if there is an easy
preparation for it?
Back to the reaction though, I am certain, in agreement with chochu3, that treatment with a strong base will yeild the alkyne via dehydrohalogenation.
The hydroboration will yeild the enol, which will tautomerize to the ketone.
I don't suppose that anyone has any references that I might use to work up the procedure and stoichiometry for these reactions?
[Edited on 3-3-2006 by Flip] |
Well thought out.
AFAIK vil mercury sulfate form spontanously when concentrated sulfuric acid is mixed with mercury.
|
|
|