| Pages:
1
2
3
4
5
6
..
14 |
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Hehe, I'm not one for microscale. The crucible only had 1/2" of stuff in it, which is on the small side for me. 
The fun thing about scientific descriptions like that is they attempt to hide excitement. It's like one of Faraday's lectures, when he was "heating a
piece of potassium metal ... [it exploded] oh, sometimes it does that... [gets another]". What could be more nonchalant than mentioning in passing
that the fume hood was levelled? 
I'll be dissolving the salt matrix and, hmm I should've poured off the lead metal while it was molten- ah well, I can do that later just as well.
Lead in anything over (II) will give off Cl2 in HCl, so yes, it should be easy to tell which it is. 
If any PbO2 was formed, it certainly isn't as acidic as I'd expect it; Cr2O3 readily forms dichromate in a chlorate melt, displacing Cl2 gas. No Cl2
was formed in this reaction. Sodium plumbate(IV) is reasonably stable, innit?
Tim
|
|
|
Rosco Bodine
Banned
Posts: 6370
Registered: 29-9-2004
Member Is Offline
Mood: analytical
|
|
some lead complex oxides of possible interest
http://dx.doi.org/10.1016/0167-2738(92)90245-K
http://dx.doi.org/10.1016/0167-2738(96)00161-0
http://dx.doi.org/10.1016/j.solidstatesciences.2007.07.004
I have requested these articles 
so hopefully they are soon available
and we can see them to learn more .
After reviewing these articles it is not encouraging that these materials would be of experimental interest outside
of a semiconductor manufacturing facility because of the
methods of grinding and pelleting , sintering and high temperatures required . So I am not attaching these references . Sorry folks , my reference
digging has turned
up no gold nuggets today although the proportional formulas
do look so interesting .
[Edited on 20-12-2007 by Rosco Bodine]
|
|
|
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
Lead Nitrate
Lead nitrate is the most soluble lead salt (55g/100g water), and is a popular choice for a plating bath when making PbO2 anodes. The following
procedure was described briefly by "tentacles" in one of the anode making threads. The method is very useful as it
does not require nitric acid. The procedure is essentially the "nitrate" version of the copper carbonate / acetic acid method described by "MadHatter" earlier in this thread for producing lead acetate (I have also used this very successfully) in that Pb is
brought into solution by electrochemical replacement by Cu.
After a few initial hiccups, I found this nitrate method to be both cheap, safe, elegant and relatively simple.
The method is based on making copper nitrate from two cheap and easily obtained agricultural chemicals (calcium nitrate and copper sulphate) and then
reacting the copper nitrate with Pb metal, to form lead nitrate.
1) Ca(NO3)2 (164.2g) + CuSO4 (159.6g) ---> Cu(NO3)2 (187.6g) + CaSO4
2) Cu(NO3)2 (187.6g) + Pb (207.2g) ---> Pb(NO3)2 (331.2g) + Cu
Equation 1 is a metathesis reaction in which CaSO4 is precipitated, and is a useful procedure for producing many different nitrates. Unfortunately it
can be difficult for the amateur as the CaSO4 precipitate is particularly thick and it can be difficult to separate from the desired nitrate solution.
In this situation, a vacuum or pressure filtration system is needed if one wants to work at reasonable concentrations. If you do not have adequate
filtration, it may be possible to do this in a stepwise fashion at lower concentrations. Ensure that you have the correct starting material, do not
use calcium ammonium nitrate (CAN) as this is an ammonium nitrate - calcium carbonate mix.
Remember blue copper sulphate is in the form of the pentahydrate, so you will require 1.56 times the stoichiometric amount in equation 1. Use a slight
excess of Ca(NO3)2 and remember that CaSO4 has slight but appreciable solubility (.21g/100g) some of this can be removed by concentrating the Cu(NO3)2
solution and cooling to 0 oC. The CaSO4 will crystallise out as very fine short aciculate crystals. If it is not removed it will react with the Pb++
to form insoluble PbSO4.
Once you have obtained your blue Cu(NO3)2 solution, place it in a beaker and hang excess strips of brushed and cleaned lead sheet in the solution.
Shot or other forms of Pb could be used, but may need stirring from time to time. Immediately, any shiny Pb surfaces will turn brown, after a minute
or so, when a strip is removed, shiny specks of Cu will be seen, these may turn brown within a few seconds of being removed from the solution. After
several minutes, a distinct plating of Cu will be observed on the Pb sheet, and it will become corroded as it goes into solution.
Leave the reaction to proceed for 24 hours in a ventilated place (some nitrogen oxides are produced). After this time all reactions should have ceased
and the solution will be a very pale green colour. Filter out all the debris and insoluble material, add a few drops of nitric acid, concentrate by
boiling and allow to cool slowly to 0 oC. The Pb(NO3)2 crystallises out as snow white, well formed, octahedral crystals.
Image shows 88g of Pb(NO3)2 (beautiful but deadly).

|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Ooh, octahedral! I love octahedra! 
Sweet! (And probably literally, like lead acetate!)
Tim
|
|
|
Aqua_Fortis_100%
Hazard to Others
  
Posts: 302
Registered: 24-12-2006
Location: Brazil
Member Is Offline
Mood: †
|
|
Really NICE!
I will surely try that in a later momment..
| Quote: | Originally posted by Xenoid:
Ensure that you have the correct starting material, do not use calcium ammonium nitrate (CAN) as this is an ammonium nitrate - calcium carbonate mix.
|
Why dont use this?
Assuming that in some places its the only avaliable OTC gardening and most next of the desired pure Ca(NO3)2.
I'm assume that one will have some work to use this , but seems to be easy , just mixing this CAN with hardware lime and bring to boil in an open
ventilated area (because of the ammonia) and let it boiling for a long time and/or when ammonia smell stops.
Even the SMDB library has an article regarding this:
http://www.sciencemadness.org/library/cano3.html
| Quote: | Leave the reaction to proceed for 24 hours in a ventilated place (some nitrogen oxides are produced). After this time all reactions should have ceased
and the solution will be a very pale green colour. Filter out all the debris and insoluble material, add a few drops of nitric acid, concentrate by
boiling and allow to cool slowly to 0 oC. The Pb(NO3)2 crystallises out as snow white, well formed, octahedral crystals.
|
Why nitrogen oxides are evolved ? It just seems to be a little weird , not (?)
It can seems like a little off-topic and I'm missing something but ,
Why lead nitrate seems to be *always* preferred over lead acetate in PbO2 anode plating?
[Edited on 25-1-2008 by Aqua_Fortis_100%]
"The secret of freedom lies in educating people, whereas the secret of tyranny is in keeping them ignorant."
|
|
|
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
| Quote: | Originally posted by Aqua_Fortis_100%
Why dont use this?
Assuming that in some places its the only avaliable OTC gardening and most next of the desired pure Ca(NO3)2.
Why nitrogen oxides are evolved ? It just seems to be a little weird , not (?)
|
What I really mean't was, don't confuse the two. There are a bewildering array of mixtures of calcium nitrate / ammonium nitrate / calcium carbonate.
These come under such names as CAN, Nitrochalk, etc. I used pure calcium nitrate from a hydroponics supplier, it was only NZ$12 for 5Kgs. If you have
to make calcium nitrate, this is a large extra step, and defeats the simplicity of the procedure somewhat!
Some nitric acid is produced as a side reaction, the pH of the solution drops to about 4 and bubbles occur around the lead. I assume with both Cu and
Pb metal in close proximity a complex electrochemical cell is formed. Perhaps someone else may be able to explain this more authoritatively.
|
|
|
Aqua_Fortis_100%
Hazard to Others
  
Posts: 302
Registered: 24-12-2006
Location: Brazil
Member Is Offline
Mood: †
|
|
Oh sorry Xenoid, I really missed what you have said ..
And really, using pure materials will be much cleaner and better!
Thank you by the practical info about this NOx generation.. Looking at first , theoretical , seems to be a "inofensive" (no poisonous gas generation ,
although poisonous liquid ).. Only seems to occur a displacement between Pb an Cu+2..So this can be dangerous since one could even consider safe to do
this indoors..
And as you said, is probably some kind of side reaction, although I have no idea how this reaction occurs..
[Edited on 25-1-2008 by Aqua_Fortis_100%]
"The secret of freedom lies in educating people, whereas the secret of tyranny is in keeping them ignorant."
|
|
|
chloric1
International Hazard
    
Posts: 1070
Registered: 8-10-2003
Location: GroupVII of the periodic table
Member Is Offline
Mood: Stoichiometrically Balanced
|
|
Xenoid-It is quite notable that you obtain such white crystals from a dirty green solution still containing copper ions. I am glad this worked out for
you.
Fellow molecular manipulator
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
I often get clear, colorless, well-formed crystals from colored solutions -- yeah, after forgetting about the solution for about a month! I am
suprised your crystals are white, why would that be? Could also be the white stuff is obscuring any green.
Tim
|
|
|
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
@ chloric1
Actually the solution was not really "dirty green". I only filtered the solution using paper towel in a sieve. I have done this procedure twice now,
the first time the solution was almost colourless, just a slight greeny blue, the second time was slightly more strongly coloured. But hey, we are not
talking grass-green here, just very pale. Given the difference in ionic radii, it's unlikely any Cu is incorporated into the Pb(NO3)2 crystalline
structure.
@ 12AX7
Lead nitrate IS white, it's not colourless. White crystals look quite wierd, actually!
[Edited on 25-1-2008 by Xenoid]
|
|
|
Xenoid
National Hazard
   
Posts: 775
Registered: 14-6-2007
Location: Springs Junction, New Zealand
Member Is Offline
Mood: Comfortably Numb
|
|
| Quote: | Originally posted by Xenoid
Lead nitrate IS white, it's not colourless. White crystals look quite wierd, actually!
|
Hmmm..! I might just have to comment on my own post here!
My description of lead nitrate being white does not appear to be correct! It was based on my own observations of lead nitrate crystals which I had
made previously from Pb and nitric acid, they also formed white (not colourless) crystals in the bottom of the beaker, they had been sitting in the
fridge for several weeks. Today I decided to extract all the lead nitrate from this preparation to add to my new supply. I redissolved the crystals in
water with a few drops of nitric acid added, and slowly cooled the saturated solution, some beautiful crystals formed. The overall appearance is
white, but many of the crystals appear perfectly clear, some have whitish cores, and some are zoned.
My chemical data book refers to lead nitrate as white, but it also says NaCl, NaClO3 and KNO3 etc. are white. In fact it doesn't differentiate between
white and colourless, so it is not much help.
I realise colourless crystals can look white due to inclusions of liquids and gases as well as fractures, etc. But the white lead nitrate crystals
previously described, are very well formed, they almost look as though they are molded from white PVC. Perhaps the presence of a little nitric acid in
the solution causes the white crystals.
I was just wondering what other people's experiences with lead nitrate have been, I wonder if I purify further will I get wholly colourless crystals.
Edit: Just recrystallising another batch and they look just whitish/colourless, nothing unusual. Wonder what caused the intense, snow white appearance
of the first crystallisation.
[Edited on 28-1-2008 by Xenoid]
|
|
|
chloric1
International Hazard
    
Posts: 1070
Registered: 8-10-2003
Location: GroupVII of the periodic table
Member Is Offline
Mood: Stoichiometrically Balanced
|
|
I second the white claim. When I prepare lead nitrate from lead and nitric acid, I always far exceed the saturation point of lead nitrate. I get a
fine granular rocksalt like white substance. I never tried to get larger crystals.
Fellow molecular manipulator
|
|
|
JohnWW
International Hazard
    
Posts: 2849
Registered: 27-7-2004
Location: New Zealand
Member Is Offline
Mood: No Mood
|
|
Lead nitrate? That is Pb(II) nitrate, and has to undergo electrolytic oxidation and hydrolysis for PbO2. Pb(NO3)4 probably cannot be isolated, but the
basic nitrate PbO(NO3)2 would probably exist as a soluble intermediate salt.
|
|
|
kilowatt
Hazard to Others
  
Posts: 322
Registered: 11-10-2007
Location: Montana
Member Is Offline
Mood: nitric
|
|
Here is my latest setup for preparing lead sulfate from scrap lead, actually for the separation of the scrap alloy into lead, tin, and antimony. I am
using a water cooled battery of 8 cells filled with 33% sulfuric acid and scrap lead electrodes in series running on about 18V AC (2.25V AC per cell),
which is somewhat equivalent to charging and discharging a lead acid cell 60 times per second. The lead sulfate rapidly falls away and collects at
the bottom of the cells, while tin sulfate remains dissolved. Higher cell voltages make it go faster but there is more loss to fuming and spatter.
As I understand it, antimony sulfate could be separated by washing the precipitates in sulfuric acid.
Larger pic here.
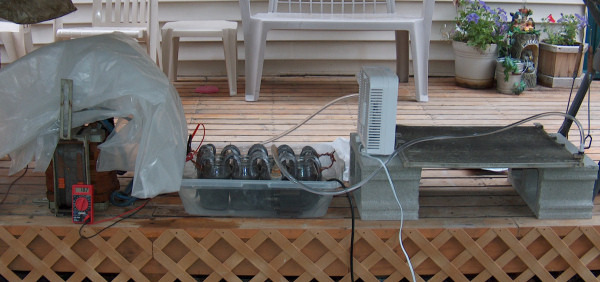
The mind cannot decide the truth; it can only find the truth.
|
|
|
tumadre
Hazard to Others
  
Posts: 171
Registered: 10-5-2005
Member Is Offline
Mood: No Mood
|
|
How much current is that? or do you prefer not to know
|
|
|
kilowatt
Hazard to Others
  
Posts: 322
Registered: 11-10-2007
Location: Montana
Member Is Offline
Mood: nitric
|
|
I couldn't find my current meter until after I posted this. I did later though, and it's about 15A.
The mind cannot decide the truth; it can only find the truth.
|
|
|
tumadre
Hazard to Others
  
Posts: 171
Registered: 10-5-2005
Member Is Offline
Mood: No Mood
|
|
well the transformer is about the right size
|
|
|
kilowatt
Hazard to Others
  
Posts: 322
Registered: 11-10-2007
Location: Montana
Member Is Offline
Mood: nitric
|
|
That's actually a pole transformer wired for 2400:480. I'm using it at less than 1/20th its rated voltage and 1/3rd its rated current for this. I've
done some fun high voltage stuff with that transformer too.
The mind cannot decide the truth; it can only find the truth.
|
|
|
Picric-A
National Hazard
   
Posts: 796
Registered: 1-5-2008
Location: England
Member Is Offline
Mood: Fuming
|
|
this is a tiny bit of the topic.
Until now i have made my lead dioxide from a 3 way process.
First i heat lead (of wich i have lots of) in a steel cruicible at around 600 degrees C. This makes Lead monoxide PbO (litharge).
Then calcination of PbO at aroung 460 degrees C produces Lead tetroxide Pb2O3.
Finally dissolve the lead tetroxide in nitric acid wich produces Lead dioxide- usefull Lead Nitrate- usefull
Pb3O4 + 4 HNO3 → PbO2 + 2 Pb(NO3)2 + 2 H2O
im now going to try the electrolosis of sulphuric acid dil using lead electrodes.
can someone tell me how i go about electroplating lead dioxide on carbon arc electrodes?
Picric-A
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Lead tetroxide is Pb3O4, which can be written as 2PbO.PbO2 (as it has lead in a mixed valence, II and IV).
|
|
|
dann2
International Hazard
    
Posts: 1523
Registered: 31-1-2007
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Picric-A
this is a tiny bit of the topic.
Until now i have made my lead dioxide from a 3 way process.
First i heat lead (of wich i have lots of) in a steel cruicible at around 600 degrees C. This makes Lead monoxide PbO (litharge).
..................
..............can someone tell me how i go about electroplating lead dioxide on carbon arc electrodes?
Picric-A |
How long do you have to heat the Lead metal to get it to convert to Lead Monoxide?
What are the dimensions of the crucible that you use to heat it (area exposed to air).
Do you keep scooping off the Monoxide as it is formed or just leave the crucible there for long enough to convert a large amount of metal?
See the 'More on PbO2 anodes' thread for some info on coating Graphite with Lead Dioxide.
Dann2
|
|
|
Picric-A
National Hazard
   
Posts: 796
Registered: 1-5-2008
Location: England
Member Is Offline
Mood: Fuming
|
|
It doesnt take long to convert to PbO however the lead forms a oxide layer wich you have to scape off occasionly. the oxide forms quickest at 600
degrees c wich is why i reccomend that however you need to scrape it off every minute or so.
My cruicible is 6cm wide by 9cm tall. becuase i have a small cruicible i have to make it in small batches
what i am aiming for next is to get a low form cruicible, say 15cm wide and 3cm high. this will avoid the need for continuous scooping of oxide layer.
hope this helps,
Picric-A
|
|
|
Picric-A
National Hazard
   
Posts: 796
Registered: 1-5-2008
Location: England
Member Is Offline
Mood: Fuming
|
|
the picrtue shows the products of roasting lead at around 600 degrees C. I didnt do it for long enough so not all the lead managed to react.
on the left is a yellowish black pile of lead oxide, Pb2O3 + PbO and on the right is puddle of unreacted lead. In front is the cruicible i used.
EDIT by Davster:
Don't rape the board formatting. I edited it for you
[Edited on 20-7-2008 by The_Davster]

|
|
|
Picric-A
National Hazard
   
Posts: 796
Registered: 1-5-2008
Location: England
Member Is Offline
Mood: Fuming
|
|
woops i think its a bit big  soz admin! soz admin!
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Yeah, way too fucking big, and horribly out of focus to boot.
|
|
|
| Pages:
1
2
3
4
5
6
..
14 |