nannah
Hazard to Others
  
Posts: 136
Registered: 20-12-2013
Member Is Offline
Mood: No Mood
|
|
What procentage is my bleach?
Hi, guys!  I have a bit of a problem. I want to figure out how much sodium
hypochlorite thats in the bottle of bleach that i bought today I have a bit of a problem. I want to figure out how much sodium
hypochlorite thats in the bottle of bleach that i bought today
On the 1 liter bottle it say that it contain 27 grams of NaClO.
I am new to this so please dont be too hard on me.  What i am thinking about
using it for making some chloroform by the Haloform reaction. I am going to do it accordingly to UC235 on youtube. What i am thinking about
using it for making some chloroform by the Haloform reaction. I am going to do it accordingly to UC235 on youtube.
Can i scale the reaction down by using only one fourth of the acetone/bleach? I havent gotten into stochiometry yet.
Thanks guys. 
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
This might be a useful link, try searching next time. A 1L bottle containing 27g of NaClO sounds rather dilute ~2.5%. Bleach is generally
between 5% and 10% sodium hypochlorite. And yes, the amounts of bleach/acetone can be scaled accordingly. Make sure that you add ethanol to
stabilize the chloroform and I would be rather careful as you don't seem to have much knowledge of chemistry(not an insult, just a warning as
chloroform can be dangerous if not synthesized properly).
|
|
|
nannah
Hazard to Others
  
Posts: 136
Registered: 20-12-2013
Member Is Offline
Mood: No Mood
|
|
Im sorry, but i couldnt find anything in the small amout of time i had. I had 5 minutes over from the kids that was clinging on me.
But thanks for the help, and for the advice. I have some 96% EtOH. And im not taking offence, i really need the practice.
I will check out the link right away.
Ps. Another thing also. I havent built a fume hood yet, but do you think it may be ok if i secure the joints properly with grease, and teflon tape?
Ps II. Would you redistill it a second time and dry it, or?
/N.
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
If you have a ground glass distillation apparatus, then you should need teflon tape, grease the joints and do it outside and you should be fine.
Distilling it twice is unnecessary, distilling it once then drying with anhydrous Na2SO4 or MgSO4 should work just fine.
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
If you're in a hurry, boil some of it down to nothing in something like a test tube, then continue heating till all of it is NaCl. T'would be a good
exercise in stiochemistry.
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
It wouldn't boil down to NaCl. It would boil down to a mixture of NaCl, NaClO3(which will eventually decompose, it will just take awhile), NaOH,
NaCO3, and surfactants, all of which may be contaminants in the bleach.
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
Quote: Originally posted by gdflp  | | It wouldn't boil down to NaCl. It would boil down to a mixture of NaCl, NaClO3(which will eventually decompose, it will just take awhile), NaOH,
NaCO3, and surfactants, all of which may be contaminants in the bleach. |
I forgot about the NaOH and NaCO3, as well as the bleach contaminants. I meant that one would continue heating until decomposition.
|
|
|
Zyklon-A
International Hazard
    
Posts: 1547
Registered: 26-11-2013
Member Is Offline
Mood: Fluorine radical
|
|
Yeah, that's a really bad idea. NaO3Cl doesn't just "slowly decompose". If pure it will probably explode, if mixed with 2 parts NaCl (by
moles) it proably wont, but why take that chance?
Also, that's not a very good way to evaluate the concentration. Add a known concentration of H2O2 and messure the weight loss of
oxygen.
nannah, you need to learn stiochoimetry, there's no way around it. It's as simple as addition in math.
|
|
|
Etaoin Shrdlu
National Hazard
   
Posts: 724
Registered: 25-12-2013
Location: Wisconsin
Member Is Offline
Mood: Insufferable
|
|
Guys, subscripts are our friends.
|
|
|
dermolotov
Hazard to Others
  
Posts: 114
Registered: 13-12-2014
Location: Toronto, Canada
Member Is Offline
Mood: Free
|
|
A simple redox titration should work. You can follow the simple thiosulphate- iodine indicator method used in undergraduate labs.
Or get creative with oxalic acid (which should act like a neutralisation reaction, too). Just do some weak acid/ base calculations and find a good
indicator.
Concentration shan't matter when performing the haloform reaction. Use excess of bleach and distil like people above me have stated.
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
OK, sorry about my lack of subscripts (gonna do it again though...)
NaO3Cl, Zyklon-A? Sorry to be on your case, but weren't you involved in the NaOCl/NaClO debate a little while back? Not that it matters, I guess, as
you could have just been busy.
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
Subscripts are easy! Just use the [ sub ] [ /sub ] tags (remove spaces). There's even a button that does it for you on the "Post Reply" window!
|
|
|
Zyklon-A
International Hazard
    
Posts: 1547
Registered: 26-11-2013
Member Is Offline
Mood: Fluorine radical
|
|
Quote: Originally posted by The Volatile Chemist  |
NaO3Cl, Zyklon-A? Sorry to be on your case, but weren't you involved in the NaOCl/NaClO debate a little while back? Not that it matters, I guess, as
you could have just been busy. |
Yes I was involved in that debate, and if you don't recall the structure is: Na+~ -O-O=Cl=O
Which is best represented with the formula NaO3Cl, unless you want to do: NaOOClO, which is more accurate still, but less useful.
The best way to write perchlorates, chlorates, chlorites and hypochlorites is XO4Cl, XO3Cl, XO2Cl and XOCl.
Most people don't do this, but it's the most accurate way.
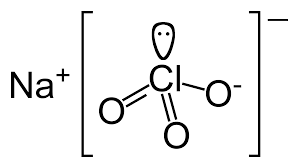
[Edited on 17-12-2014 by Zyklon-A]
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
OK. I don't agree, but it's totally opinionated, and I don't recall what the IUPAC states about it.
|
|
|
j_sum1
Administrator
       
Posts: 6218
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
I agree. But there are gazillions of posts here where they are not used. Why bring it up now?
Besides it is a PITA to do sometimes. Compare H2SO4 and H2SO4 on my phone. That's ten keytaps compared with 56. If everyone
understands, it's not ambiguous and is consistent with the culture of the board and is used by mods and admins then what's the harm?
|
|
|