azoth
Harmless

Posts: 5
Registered: 2-4-2006
Member Is Offline
Mood: No Mood
|
|
Question About Electrochem Technique/Apparatus
I am thinking, well more than just thinkingl, about trying some various electrochemistry experiments, and I've gotten most of the pieces i need
together to do them.
the one thing i'm having problems with is finding a suitable cooling coil, that i can use in tandem with a circulating water pump.
i know a glass coil, with the right dimensions. would be ideal, but this could run in the hundreds of dollars,
i want to fit up to 1/2" electrodes between the coils, and don't want the coils to have a larger total diameter more than 2" so that it can fit in the
cell.
does any electrochemists out there have any tips on DIY coil tubing materials or whatnot, to make an effective recirculated water cooling 'coil'?
im fresh out of ideas.
oh and 5 or so revolutions or 'coils' would be sufficient for the cooling needs i have planned.
Thanks!
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Copper? Stainless? No good for reactive solutions of course. How about polypropylene tubing? Plastic isn't real conductive yeah, but you can look
for thin tubing.
Tim
|
|
|
azoth
Harmless

Posts: 5
Registered: 2-4-2006
Member Is Offline
Mood: No Mood
|
|
i dont' think copper of stainless steel would work too well, since they are conductive.
i've thought of the tygon tubing route, but some of the cathode/anode solutions will be fairly concentrated acids and stuff, and i've soaked a few in
them and they seem to 'leach' over time,
although i suppose i could make a bunch of disposable tygon coils, but its a bitch to try and make them sturdy like glass would be.
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
Just use an ice water bath for the cell, adding more ice water when necessary. I do a fair bit of electrochem, and when something needs cooling this
is what I do.
|
|
|
azoth
Harmless

Posts: 5
Registered: 2-4-2006
Member Is Offline
Mood: No Mood
|
|
yeah. but it's a cell within a cell.
so an ice bath really won't work very efficiently as far as heat/cooling transfer.
|
|
|
Twospoons
International Hazard
    
Posts: 1282
Registered: 26-7-2004
Location: Middle Earth
Member Is Offline
Mood: A trace of hope...
|
|
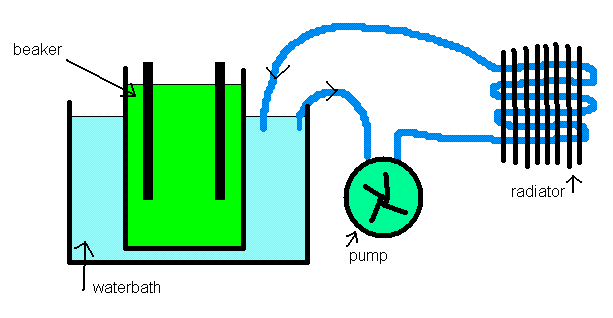
If your inner cell is a thin walled glass beaker, you should get plenty of heat transfer to a waterbath. You can then pump your water bath water
through another external cooling system to keep the bath temp down.
Heat transfer out of your electrolyte will also improve if it is stirred.
Helicopter: "helico" -> spiral, "pter" -> with wings
|
|
|