stygian
Hazard to Others
  
Posts: 242
Registered: 19-9-2004
Member Is Offline
Mood: No Mood
|
|
keto-enol question
If you have a ketone like MEK, would the enol form be:
CH3C(OH)CHCH3 or CH2C(OH)CH2CH3 ?
|
|
|
MassConfusionSquared
Harmless

Posts: 3
Registered: 16-3-2006
Member Is Offline
Mood: unenlightened
|
|
Both forms would theoretically exists but the more highly substituted carbon would be more acidic, giving up it's proton for a pair of electrons.
H3C-(C-OH)=CH-CH3
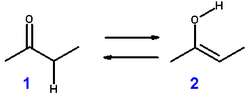
|
|
|