| Pages:
1
2 |
Krypton
Hazard to Self
 
Posts: 90
Registered: 21-11-2002
Location: Spain
Member Is Offline
Mood: explosive 21
|
|
new ernergetic materials
I think the nitration of pentazole gave a strong energetic material.
N5-NO2
for instance
3-nitro-1,2,3,4,5-cyclopentazole

In convention with my ancestor.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
What made you think that?
Cyclopentazole should be CN5 squeleton!
The possible nitration in 3 would mean that C has 2 H and one of the N has 1:
CH2(-N=N-)2NH -HNO3-> CH2(-N=N-)2N-NO2 + H2O
(O2N)2C(-N=N-)2NH
O2N-CH(-N=N-)2NH
O2N-CH(-N=N-)2N-NO2
(O2N)2C(-N=N-)2N-NO2
Should then be also investigated!
  
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Nick F
Hazard to Others
  
Posts: 439
Registered: 7-9-2002
Member Is Offline
Mood: No Mood
|
|
5 membered nitrogen rings do not exist (yet  ), except the pentazole ion ), except the pentazole ion  . Even that has only been isolated as a compund with AsF6-, I think, and that exploded
while they were carrying out tests on it. You might want to check that, there may have been more developments but that was the last that I heard on
the subject. . Even that has only been isolated as a compund with AsF6-, I think, and that exploded
while they were carrying out tests on it. You might want to check that, there may have been more developments but that was the last that I heard on
the subject.
Tetrazole exists, I have 2kg of 5-aminotetrazole. Even on it's own it is quite energetic, if you heat some up on a spoon after a short while it
will start a fairly violent self-sustaining decomposition.
The nitrate salt is really good stuff, it is the only nitrate salt I've encountered that is easy to set off with a hammer blow  . I would guess that it would be powerful compared to other nitrates, too. A great
use for it is 5-ATZN dynamite, but I don't make it too often since I have not found any safety data for the nitrate. Also when I make a batch of
5-ATZN I end up burning most of it since it makes a really nice flame . I would guess that it would be powerful compared to other nitrates, too. A great
use for it is 5-ATZN dynamite, but I don't make it too often since I have not found any safety data for the nitrate. Also when I make a batch of
5-ATZN I end up burning most of it since it makes a really nice flame  . .
|
|
|
Krypton
Hazard to Self
 
Posts: 90
Registered: 21-11-2002
Location: Spain
Member Is Offline
Mood: explosive 21
|
|
(>N=N=N=N=N< -(NO2) -(NO2)
|
|
|
Krypton
Hazard to Self
 
Posts: 90
Registered: 21-11-2002
Location: Spain
Member Is Offline
Mood: explosive 21
|
|
Here a little draw of nitropentazole.
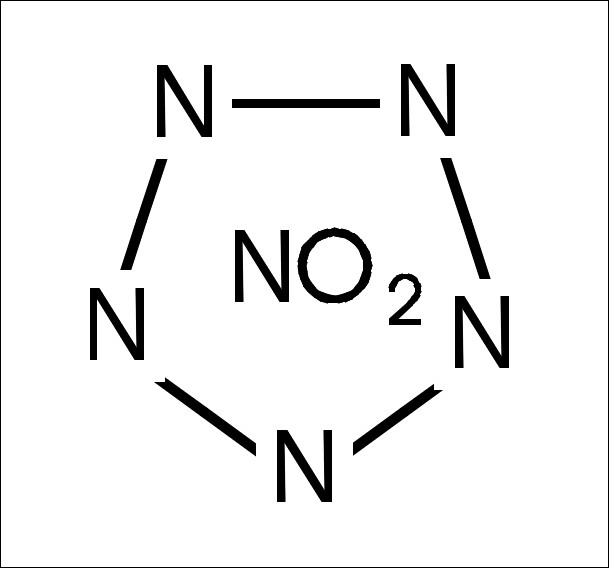
In convention with my ancestor.
|
|
|
BASF
Hazard to Others
  
Posts: 282
Registered: 5-11-2002
Member Is Offline
Mood: hydrophilic
|
|
| Quote: |
Tetrazole exists, I have 2kg of 5-aminotetrazole. Even on it's own it is quite energetic, if you heat some up on a spoon after a short while it
will start a fairly violent self-sustaining decomposition.
|
Wow!
Where did you get that stuff?
- You must have been selling your home for that quantity i guess....
Sigma-Aldrich??
Really keen, HLR
|
|
|
madscientist
National Hazard
   
Posts: 962
Registered: 19-5-2002
Location: American Midwest
Member Is Offline
Mood: pyrophoric
|
|
Nick, you should try oxidizing the amino group in your 5-aminotetrazole to a nitro group, ozone being the oxidant. Just imagine 5-nitrotetrazole! 
I weep at the sight of flaming acetic anhydride.
|
|
|
Nick F
Hazard to Others
  
Posts: 439
Registered: 7-9-2002
Member Is Offline
Mood: No Mood
|
|
I would rather not reveal my exact source, but I will say that a bit of searching on the net and a few nice e-mails can get you a lot of nice things
     . .
The original plan was to make 5-aminotetrazole dinitramide, N4HCNH3(+) (-)N(NO2)2, but without dry ice (ie, using my freezer which gets to around
-22*C) the yield of KDN that I've been getting is hardly worth bothering to make, and I don't get dry ice very often.
HNO2 turns it into ditetrazolyltriazine, which seems surprisingly stable (the sample I made is still here, and still seems normal...), but all
oxidising agents I've tried so far turn it into a load of bubbles  . .
|
|
|
Krypton
Hazard to Self
 
Posts: 90
Registered: 21-11-2002
Location: Spain
Member Is Offline
Mood: explosive 21
|
|
tetrazoles
Why ditetrazoyltriazine and not 2,4,6-tritetrazoyl-1,3,5-triazine ?
A other examble of the tetrazine family is
3,6-bis(3,5-diaminopyrazoyl)-1,2,4,5-tetrazine a stable expensive secondary explosive.
In convention with my ancestor.
|
|
|
Krypton
Hazard to Self
 
Posts: 90
Registered: 21-11-2002
Location: Spain
Member Is Offline
Mood: explosive 21
|
|
sorry, a little copy mistake in my last post.
The correct name of course
3,6-bis(3,5-diaminotetrazolyl)-1,2,4,5-tetrazine
In convention with my ancestor.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Ok! I see!
N5-NO2 thus nitrocyclopentaazapentadiene!
(-N=N-)2N-NO2
NH2-CHN4 -->
perchlorate NH2-CHN4.HClO4
nitrate NH2-CHN4.HNO3
nitroformate NH2-CHN4.HC(NO2)3
Whatabang!

PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Krypton
Hazard to Self
 
Posts: 90
Registered: 21-11-2002
Location: Spain
Member Is Offline
Mood: explosive 21
|
|
Why not
pentanitrocyclopentaazapentadiene
>NNO2-NNO2-NNO2-NNO2-NNO2->
or
pentanitrocyclopentaazapentadienecyclopentadiene
[{5[(-N-N-)2N-NO2]}C5H]
the topic question
was nitrocyclopentazole
and not
nitropentazole
    
In convention with my ancestor.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
>pentanitrocyclopentaazapentadiene >NNO2-NNO2-NNO2-NNO2-NNO2-
-This is pentanitrocyclopentaazapentane!
No dienicdiaza -N=N- here!
>pentanitrocyclopentaazapentadienecyclopentadi>ene >[{5[(-N-N-)2N-NO2]}C5H]
-Could you make a drawing, I think there is something wrong here  
>the topic question was nitrocyclopentazole >and not nitropentazole
-Unless I'm wrong you wrote in the first post:
"I think the nitration of pentazole gave a strong energetic material. N5-NO2
for instance 3-nitro-1,2,3,4,5-cyclopentazole"
I don't know what to think or to answer, you are rather confusing      
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Krypton
Hazard to Self
 
Posts: 90
Registered: 21-11-2002
Location: Spain
Member Is Offline
Mood: explosive 21
|
|

When your cyclopentaazapentadiene nitrated with five than the nito name
cyclopentaazapentadienepentanitrate
and the formula name
1,2,3,4,5-pentanitrocyclopentazole
and your aza name
1,2,3,4,5-pentanitro-1,2,3,4,5-pentazacyclopentane
    
The draw to the double-cyclo-compound later !!
In convention with my ancestor.
|
|
|
Krypton
Hazard to Self
 
Posts: 90
Registered: 21-11-2002
Location: Spain
Member Is Offline
Mood: explosive 21
|
|
I`ve upload a picture to
ftp://ftp.sciencemadness.org of
1,2,3,4,5-pentanitro-1,2,3,4,5-pentazacyclopentane
with the double bound of the nitroaza to the cyclopropane.
In convention with my ancestor.
|
|
|
Krypton
Hazard to Self
 
Posts: 90
Registered: 21-11-2002
Location: Spain
Member Is Offline
Mood: explosive 21
|
|
it`s of course the
double bound of the nitroaza to the cyclopentane and not cyclopropane !!!
In convention with my ancestor.
|
|
|
Krypton
Hazard to Self
 
Posts: 90
Registered: 21-11-2002
Location: Spain
Member Is Offline
Mood: explosive 21
|
|
examples
I`ve uploaded some example of non-caged
cyclo n5 and c5 components and their combinations
to ftp.
penta(nitrocyclopentazol)-cyclopentadiene
penta(3-nitrocyclopentaazacyclopentaene)-cyclopentane
cyclo[penta(3-nitrocyclopentazol)-cyclopentane]
I don`t know, the electron bounds in example 1 can be true with 4 ????
In convention with my ancestor.
|
|
|
BASF
Hazard to Others
  
Posts: 282
Registered: 5-11-2002
Member Is Offline
Mood: hydrophilic
|
|
bromo-RDX?
I wonder wether it would be possible to brominate RDX...
Mild conditions.
The problem is, i know few to nothing about nitramine-chemistry.....
This could yield an explosive with zero to positive oxygen-balance and an incredible density.
The stability could be still good if bromine is used instead of chlorine, which is also less selective.
I mention this idea especially because RDX is within reach without using acetic anhydride, bromination should also be easy.
I have a procedure on how to free bromine from bromides, the stage of reaction can be monitored by the coulor of the solution.
When the bromine reacts, the solution slowly decoulorizes.
HLR
[Edited on 7-5-2003 by BASF]
|
|
|
Krypton
Hazard to Self
 
Posts: 90
Registered: 21-11-2002
Location: Spain
Member Is Offline
Mood: explosive 21
|
|
BASF,
What will you do with this stuff ?
Something like explosives for grandmothers.
In convention with my ancestor.
|
|
|
BASF
Hazard to Others
  
Posts: 282
Registered: 5-11-2002
Member Is Offline
Mood: hydrophilic
|
|
I still prefer realizable ideas.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Good idea BASF!
halogenation of RDX might work but as you said the C-N link might not survive the halide.
Anyway the halide will have higher densities and must then because their OB is improved lead to higher brisance and VOD performances.
(-CHX-N(NO2)-)3 where X = F, Cl, Br or I
Those haloRDX are also interesting because they might be precursor for couplage reactions of other explosophoric groups.
Maybe a different way of action must be investigated!
CH3-O-CH=O + NH2-NO2 --> CH3-O-CH=N-NO2
3CH3-O-CH=N-NO2 --> (-CH(OCH3)-N(NO2)-)3
(-CH(OCH3)-N(NO2)-)3 +3 HBr(g) --> (-CH(OH)-N(NO2)-)3 + CH3Br(g) or (-CHBr-N(NO2)-)3 + CH3OH
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Krypton
Hazard to Self
 
Posts: 90
Registered: 21-11-2002
Location: Spain
Member Is Offline
Mood: explosive 21
|
|
Bromination !
http://www.hanson.btinternet.co.uk/g0007.htm
In convention with my ancestor.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Sorry to say but genealogy has nothing to do with bromination.
Unless it was a bad taste joke     , you have made a mistake in the link! , you have made a mistake in the link!
   
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
Krypton
Hazard to Self
 
Posts: 90
Registered: 21-11-2002
Location: Spain
Member Is Offline
Mood: explosive 21
|
|
This was a insinuation to some members in the forum which scattering some topic themes with crap.  
In convention with my ancestor.
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Sabotage on sciencemadness?
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
| Pages:
1
2 |