| Pages:
1
2
3 |
Dan Vizine
National Hazard
   
Posts: 628
Registered: 4-4-2014
Location: Tonawanda, New York
Member Is Offline
Mood: High Resistance
|
|
Barium Animus
A while back, I constructed a purge type glovebox. That kind has no purification train, and is like a glorified glovebag. It's 30 gal in vol. and the
other day I started it up for the first time. After purging with ~ 50 cubic feet of UHP argon, I set about on two tasks. The first was ampouling
lithium, the second was ampouling barium.
The atmosphere inside the glovebox was sufficiently good to allow preparation of good lithium samples. One type was high-purity pellets, another was
chunks carved out of oxidized ingots.
So, now I was ready to find out if my expectation that barium was going to be much more challenging than lithium was correct. It was. An atmosphere
capable of good handling of lithium was not good enough for barium. Barium is used as a "getter" in vacuum tubes, so small wonder. The barium could be
sanded or filed to a silver surface. This turned to a golden color within 2 to 3 seconds. This further aged to a purplish or bluish silver in the time
it took to mechanically trim each edge. The faces of the slab were quickly re-cleaned and it was put into a sealed ampoule which was flushed with more
UHP argon. The sealed vial is shown below for comparison. It's clearly metallic, it was a degree of luster, although it's muted and purplish.
I'm convinced that tumbling, under argon, in a sealed ball mill, or peanut butter jar, has promise of nicer samples. Cleaning has got to be simplified
to exclude even the cleaning process operating across the air/protective medium interface. Simplified to just grabbing the sample, dropping it into an
ampoule and evacuating or inerting.
   
"All Your Children Are Poor Unfortunate Victims of Lies You Believe, a Plague Upon Your Ignorance that Keeps the Youth from the Truth They
Deserve"...F. Zappa
|
|
|
j_sum1
Administrator
       
Posts: 6221
Registered: 4-10-2014
Location: Unmoved
Member Is Online
Mood: Organised
|
|
Those lithium ampoules are beautiful!
Are you selling them?
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Beautiful Dan.
That golden hue is so reminiscent of slightly oxidised Cs, no?
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
How do you seal ampoules in an inert atmosphere? Or do you seal outside the glove box?
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
Dan Vizine
National Hazard
   
Posts: 628
Registered: 4-4-2014
Location: Tonawanda, New York
Member Is Offline
Mood: High Resistance
|
|
Hi j_sum1,
The ampoules with the small shot were prepared for GalliumSource.com. The ampoules with the chunks are available on e-Bay right now. If you or anyone
from this site should buy one, mention it in a note and I'll refund $5. Or, just skip eBay altogether and I'll subtract $10.
Hi blogfast,
I had the exact same thought when I saw it. It's also not too dissimilar to watching a piece of polished steel that gets heated in air. Gold at first,
but then that blue and brown grows in.! I think that barium may possibly be the most difficult metal to prepare in shiny silver form.
Hi Bert,
I use rubber septa to stopper the tubes, remove them from the glovebox and then seal them.
"All Your Children Are Poor Unfortunate Victims of Lies You Believe, a Plague Upon Your Ignorance that Keeps the Youth from the Truth They
Deserve"...F. Zappa
|
|
|
Dan Vizine
National Hazard
   
Posts: 628
Registered: 4-4-2014
Location: Tonawanda, New York
Member Is Offline
Mood: High Resistance
|
|
Well, no sense in internally debating this any further. I put this together from VCR, car, printer & toy parts. I ordered a pound of SS 302 shot.
In less than a week, we'll see if this has any merit.

"All Your Children Are Poor Unfortunate Victims of Lies You Believe, a Plague Upon Your Ignorance that Keeps the Youth from the Truth They
Deserve"...F. Zappa
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Ti I've also seen with this golden sheen. Presumably it's an oxide coating only a few atoms thick.
|
|
|
argyrium
Hazard to Others
  
Posts: 123
Registered: 3-2-2008
Location: Pacific
Member Is Offline
Mood: No Mood
|
|
Very nice work, Dan. Thank you for posting for all of us to see.
And, BTW, the first photo of the sealed ampules would look very nice on a Christmas tree!
[Edited on 4-1-2015 by argyrium]
|
|
|
Dan Vizine
National Hazard
   
Posts: 628
Registered: 4-4-2014
Location: Tonawanda, New York
Member Is Offline
Mood: High Resistance
|
|
Thanks, argyrium.
Well, here's the first update on this ongoing attempt to prepare silvery barium metal via tumbling. It seems there's more to the construction of an
efficient ball mill than I realized, and a subtle balance of forces determines the outcome (speed, tilt, media drag and weight, shapes of media and
workpieces, etc.) . It was my intention to try small SS 304 pellets. I made a bunch from some ~0.2 inch diameter wire and threw 3 of the God-awful
filthiest, largely black and crusty pennies I could find in there and spun the mill up. The media refused to climb the glass wall, too smooth. Let me
take a second to state the [now] obvious...the media needs to climb as high on the revolving wall as possible, ideally succumbing to gravity near the
top, in order to fall the greatest distance to impact the workpiece. A rubber wall lining fixed this. Second run was three+ hours long and it took one
of the [not pictured] mystery-encrusted specimens to the single partially-cleaned picture seen below. Interestingly, the SS pellets were now polished
and shiny compared to their original form, below. I decided to investigate another media. I hammered some old fused alumina into pea-sized chunks and
strained out fines and added a putrid nickel. About 60 minutes of milling [with no SS pellets present] gave very dusty samples which rinsed off to
give the cleanest sample below. These were short runs, but so far I believe:
1) Hard samples clean better than soft ones.
2) Alumina is many times faster than SS. A mixture deserves a look.
3) Sample geometry is important. The round coins often rolled along the inside walls and avoided many impacts.
4) Since hardness matters, a good stand-in for Ba is needed. Pb is nearly as hard and so I made some rectangular blocks to approximate the barium
(below).
Back to the tests....
      
[Edited on 8-1-2015 by Dan Vizine]
"All Your Children Are Poor Unfortunate Victims of Lies You Believe, a Plague Upon Your Ignorance that Keeps the Youth from the Truth They
Deserve"...F. Zappa
|
|
|
phlogiston
International Hazard
    
Posts: 1376
Registered: 26-4-2008
Location: Neon Thorium Erbium Lanthanum Neodymium Sulphur
Member Is Offline
Mood: pyrophoric
|
|
Typically, the best speed to run a ball mill at is 65-75% of the 'critical speed', which is the speed at which the media is not tumbling but held
fixed against the wall all the way round by centrifugal force.
There exists a simple formula that relates the media size to the ball mill container size, which (since it appears you tend to plan experiments really
well beforehand) you most likely have found out already.
You may also want to look into using a mixture of media of different sizes.
-----
"If a rocket goes up, who cares where it comes down, that's not my concern said Wernher von Braun" - Tom Lehrer |
|
|
Dan Vizine
National Hazard
   
Posts: 628
Registered: 4-4-2014
Location: Tonawanda, New York
Member Is Offline
Mood: High Resistance
|
|
Actually, if I planned well enough, I wouldn't be re-designing now. The prototype was really supposed to be a proof-of-concept, so I guess that's
nearly an utter failure, so far. I should really try to find out if ball milling is even applicable to softer metals. Just maybe taking a small
motorized ss wheel into the gb and working harder to maintain low residual gasses in the atmosphere will have to be the answer.
Edit: Tumbling Pb with alumina chunks is a dog. The results are shown below.
Tumbling with SS in progress, expectations aren't high. I suspect a much softer medium may be needed, or perhaps just a smaller one, like dry sand.
[Edited on 8-1-2015 by Dan Vizine]

"All Your Children Are Poor Unfortunate Victims of Lies You Believe, a Plague Upon Your Ignorance that Keeps the Youth from the Truth They
Deserve"...F. Zappa
|
|
|
halogen
Hazard to Others
  
Posts: 372
Registered: 18-4-2004
Member Is Offline
Mood: No Mood
|
|
Barium you claim was used as a getter. Could you not use a sacrificial amount to... get? Not efficient enough, or too slow I guess. Just an idea.
Another, you worked inside a glove box. Purged but not perfect. What about a glove balloon? Not a idealized spherical ensemble, but inflatable
nonetheless? Squeeze out the oxygen first. I read PVDC has desirable barrier qualities.
F. de Lalande and M. Prud'homme showed that a mixture of boric oxide and sodium chloride is decomposed in a stream of dry air or oxygen at a red heat
with the evolution of chlorine.
|
|
|
Dan Vizine
National Hazard
   
Posts: 628
Registered: 4-4-2014
Location: Tonawanda, New York
Member Is Offline
Mood: High Resistance
|
|
Actually, the first layers rubbed off the barium were the initial impurities and the sacrificial layer. One problem may just be surface morphology as
the poor looking Pb sample became the below just by burnishing with no material removal.
With additional purging, the gb will reach the same purity as UHP gas, about 5 ppm O2. This is the way to go. It will be quite dry as it passes
through a 30 inch zeolite column before the box. The box is sealed on an order equaling commercial units. If I pump the gloves up at night, they are
still up 24 hours later. But, why not? I have almost no leakage points, no doors, no KF-40 ports, etc.

"All Your Children Are Poor Unfortunate Victims of Lies You Believe, a Plague Upon Your Ignorance that Keeps the Youth from the Truth They
Deserve"...F. Zappa
|
|
|
Molecular Manipulations
Hazard to Others
  
Posts: 447
Registered: 17-12-2014
Location: The Garden of Eden
Member Is Offline
Mood: High on forbidden fruit
|
|
Nice pictures Dan.
Why not run the argon through a "getter" first?
A tube with lithium turnings will work fine.
Is the impurity nitrogen or oxygen, or both?
[Edited on 8-1-2015 by Molecular Manipulations]
-The manipulator
We are all here on earth to help others; what on earth the others are here for I don't know. -W. H. Auden
|
|
|
Dan Vizine
National Hazard
   
Posts: 628
Registered: 4-4-2014
Location: Tonawanda, New York
Member Is Offline
Mood: High Resistance
|
|
The likely impurities are 2 ppm O2, 2 ppm (theoretical min), a few ppm N2. Unless H2O exceeds 5 ppm, Li is immune to O2 and N2.
Tumbling experiments have been many and varied. All failed.
A small motorized SS wire brush and a better atmosphere are now the intended route.
"All Your Children Are Poor Unfortunate Victims of Lies You Believe, a Plague Upon Your Ignorance that Keeps the Youth from the Truth They
Deserve"...F. Zappa
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
From a past life where I worked on very high vacuum systems:
We used Ti to scavenge the last tiny bits of gas. It reacts with both N2 and O2 QUITE well when hot-
The systems I helped build had little heated pots of Ti set up with a charge on them, with a charged grid to accelerate stray gas molecules in the
correct direction to hit the Ti, physically STICK into the surface and react there. Yours truly built the power supplies and fabricated the
electronics chassis.
Ion pump
[Edited on 13-1-2015 by Bert]
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
Dan Vizine
National Hazard
   
Posts: 628
Registered: 4-4-2014
Location: Tonawanda, New York
Member Is Offline
Mood: High Resistance
|
|
Hi Bert and Molecular Manipulations,
I can fully appreciate everything you're saying, I've been considering a lower tech form of scrubbing the atmosphere, simply stirring a small beaker
of liquid sodium potassium alloy in the glovebox before use should scavenge oxygen and moisture. But they won't touch certain other gases, like
nitrogen.
Lithium is certainly an interesting scavenger choice as it reacts so readily with nitrogen, and you might think that an atmosphere that had been
exposed to freshly cut lithium pieces for five consecutive hours (without them darkening at all) might be clean enough, but it isn't. Not by a long
shot. If I had to make a very rough estimate as to the time it takes a sample of the two freshly cut metals to be completely covered with a darker
coating I would have to say that darkening is at least 10,000 times faster for barium versus lithium. The barium went from silver to gold in a couple
seconds, and blue/brown shortly after that. Lithium remained utterly shiny in my glovebox atmosphere for the five hours I was working. I am utterly
astounded by this material. It is far more sensitive to atmospheric contamination than liquid cesium, rubidium, potassium, sodium, lithium.
I think the dark color on the barium is probably less indicative of oxygen than something else, barium left in the air turns primarily white.
And the white elephant in the room, that I haven't mentioned yet, is the fact that when you commission a new glovebox, the copper adsorbent is really
quite quick and efficient at removing oxygen. A glovebox can be started one day, and by the next day you are at sub part per million levels of oxygen.
You can even just purge down to a few ppm O2 without circulation with a liberally available gas supply, like LO2. This isn't the case for water, not
by a long shot. It has taken anywhere from 2 to 3 to as many as five days of continuous circulation before all of the adsorbed water on anything
you've put into the glovebox, and the glovebox walls themselves, has desorbed in order to be scavenged.
I know that my glovebox has an absolutely exceptional leakage rate. It's better than commercial units. I can pump the gloves up and two days later
they have not sagged, I'm not saying I have the most superior technology in the world, merely that my glovebox has very few openings to the outside
world. My supply gas is what it is, the only improvement I could make would be pretreating it with powdered barium . But even if I did that, without a
glovebox that operates continuously long enough to get dry (< a few tenths of a ppm) inside, maybe I'm just banging my head against the wall. I
can't believe I'm being defeated by a group 2 element.
Incidentally, the use of a rotating stainless steel wire brush was not particularly effective at all. It was almost as though the grooves the wires
cut reacted faster. Larger surface area. It certainly gave worse results than sandpaper or a file. Since metals generally become more tarnish
resistant the more smooth the surface is, perhaps fine grade sandpaper will at least give a surface that darkens at a slow enough rate so that I can
get samples that are only silver and gold into a vial. I don't harbor any illusions that I am going to come up with brilliant silver barium metal
anymore.
This is proving to be a real challenge. The current score:
Barium 2
Dan 0
[Edited on 21-1-2015 by Dan Vizine]
"All Your Children Are Poor Unfortunate Victims of Lies You Believe, a Plague Upon Your Ignorance that Keeps the Youth from the Truth They
Deserve"...F. Zappa
|
|
|
Dan Vizine
National Hazard
   
Posts: 628
Registered: 4-4-2014
Location: Tonawanda, New York
Member Is Offline
Mood: High Resistance
|
|
Probably, if I ground up a bunch of barium and let a large tray sit in the middle of the glovebox overnight or longer with the box at maybe 5 or 6
mbar argon and with a small circulation fan, it might make the atmosphere good enough.
This isn't over, Barium.
"All Your Children Are Poor Unfortunate Victims of Lies You Believe, a Plague Upon Your Ignorance that Keeps the Youth from the Truth They
Deserve"...F. Zappa
|
|
|
Molecular Manipulations
Hazard to Others
  
Posts: 447
Registered: 17-12-2014
Location: The Garden of Eden
Member Is Offline
Mood: High on forbidden fruit
|
|
| Quote: | Originally posted by Dan Vizine
Li is immune to O2 and N2. |
Oxygen yes, forgot about that, but it reacts readily with nitrogen, or must water be present for that as well?
Heated lithium should work, it reacts with oxygen and nitrogen, and is much cheaper than barium.
I would bet that the main impurity is nitrogen, just like lithium nitride is dark but the oxide is colorless, barium is probably similar. Of course
just because nitrogen is present doesn't mean oxygen isn't.
Either way, I suggest running the argon through a tube of hot lithium turnings/bubbling it through liquid lithium before it enters the
glovebox. That would be faster and more efficient no? Or would that be too hard to incorporate in the system?
I'm shocked that it reacts that fast in such conditions,
truly amazing. It reminds me of when I tried to make barium oxide.
I heated barium nitrate to red hot for 20 minutes until it stopped evolving nitric oxide and oxygen, then quickly crushed the fused mass and ampuled
it under nitrogen while still hot, within 40 seconds. I made three ampules this way, and tested one the next day. Turned out, based on pH tests and
quantity of carbon dioxide evolution upon reaction with acids, it was at least 90% barium carbonate.
Still haven't tried that again.
-The manipulator
We are all here on earth to help others; what on earth the others are here for I don't know. -W. H. Auden
|
|
|
Dan Vizine
National Hazard
   
Posts: 628
Registered: 4-4-2014
Location: Tonawanda, New York
Member Is Offline
Mood: High Resistance
|
|
Quote: Originally posted by Dan Vizine  | | A glovebox can be started one day, and by the next day you are at sub part per million levels of oxygen. You can even just purge down to a few ppm O2
without circulation with a liberally available gas supply, like LO2. |
No it can't. LO2 would be a poor choice. How about LN2, you senile bastard?
"All Your Children Are Poor Unfortunate Victims of Lies You Believe, a Plague Upon Your Ignorance that Keeps the Youth from the Truth They
Deserve"...F. Zappa
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
"Ti, nymphomaniac of metals. Get it hot, and it will combine with ANYTHING!"
If you're going to try Ti, you will need to heat it - Pretty non reactive stuff to atmosphere at STP. Even the finest pyro grades I have seen offered
for use in igniters can be (relatively!) safely opened in atmosphere.
Powdered Zirconium is a different kettle of fire! Packed in water, it can settle to the point where so little water remains between the particles it
is pyrophoric immediately on being scooped out of the jar.
As near as I can recall? Our high vacuum cleaning procedure was:
Start with large rotary pumps, proceed to special high vacuum pumps. With a good vacuum in the chamber, de-sorb gas & contaminants from walls: We
would bake walls with heat tapes applied to exterior and the whole outfit wrapped in insulation, heating while using an ion pump to "get" whatever
molecules came off the interior walls-
What's the glove box made of? Can you bake it...
[Edited on 23-1-2015 by Bert]
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
Praxichys
International Hazard
    
Posts: 1063
Registered: 31-7-2013
Location: Detroit, Michigan, USA
Member Is Offline
Mood: Coprecipitated
|
|
Maybe make a big coil of Ti wire (lots on eBay) and hook it to a variac. Let that sit red-hot for a while and see if that does anything. Just keep
that hood cool.
Maybe build a scrubbing unit with a blower, the titanium element, and then a gas cooler. Putting a few kV DC bias between a screen aft of the blower
and the titanium coil could help electrostatically capture a lot of stuff too.
Just some thoughts.
|
|
|
Dan Vizine
National Hazard
   
Posts: 628
Registered: 4-4-2014
Location: Tonawanda, New York
Member Is Offline
Mood: High Resistance
|
|
Bert and Praxichys,
All great suggestions and ideas. It's the kind of quality stuff I've come to really enjoy from you guys, along with blogfast 25's thoughts and some
others.
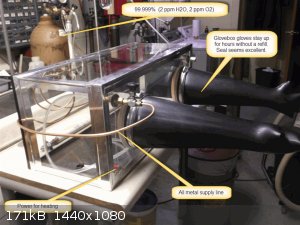
My glovebox is glass, with polyurethane, PVC, and silicones included. Gentle prolonged baking is possible. In fact, let me show you what I'm working
with:
A 20 or 30 gal fish tank was purchased for the main glovebox body because they don't leak, by definition. A sheet of 1/4" polycarbonate (Lexan) was
cut to fit the top opening snugly. All interior joints (and exterior too, for the plastic top rim anyway) are sealed with continuous beads of
silicone or polyurethane sealant. Metal tape (the only gas impermeable tape) then goes over all joints as insurance. Any wrinkles form potential
leakage pathways, so be meticulous. You should note that silicone and polyurethane adhesives and sealants are not 100% resistant to diffusion of
oxygen. That's why I used the metal tape over them anywhere possible. Otherwise, thick applications are beneficial and the best you can practically
do.
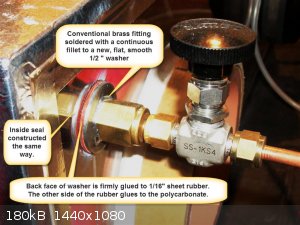
There are no doors, loading and unloading is done through a gloveport. The gas connectors that run through the Lexan have large flat washers soldered
to them in a leak proof fashion. The back sides of the washers are fitted with rubber gaskets and everything is sandwiched together and tightened down
while the silicone adhesive is still wet, silicone is also applied between the rubber washer and the Lexan. There are a total of seven holes in the
Lexan, two for electricity, two for gloveports and three for gasses.
I've given different estimates as to how long the gloves will remain up, if you're glovebox user, former or present, you know exactly what I'm talking
about, except that commercial glove boxes open their little electronic solenoids to let gases in to maintain pressure a number of times per hour. You
also know that the frequency of refill is somewhat of a good indicator of the leakage rate of the glovebox. Now, obviously I cannot measure my
glovebox pressure without instrumentation. The most accurate way I can detect significant change is to inflate the gloves it till they are just
self-supporting, not too much more. You know, sticking straight out but not fat.
The longest I've tried this test is for two days, and there was no sagging. That being said, my basement is not exactly a thermostatically controlled
environment, but I guess you'd have to say this is a pretty good leakage rate. I am somewhat amazed that the scrap pieces of lithium that I got when I
was working in the glovebox while it was in its best shape, and which were simply dropped into a glass bottle that has a all metal top and sealed with
about 10 layers of Parafilm, are still bright all these many days later. Admittedly, I once thought lithium was a high benchmark, while now I realize
it's mediocre. As long as I don't need to open that bottle it will be very interesting to see how long the shine lasts.
A note about the gloveport construction. The PVC pipes of an appropriate diameter were cut a few inches long and sealed into the polycarbonate with
polyurethane adhesive. Then the outside edge of the polyurethane tube which protruded outwards was given a layer of one quarter inch gum rubber whose
ends were firmly attached together in a butt joint with cyanoacrylate. This band was stretched and glued on to the PVC pipe. Gloves go on over this.
Don't make the mistake of putting a worm-drive gear clamp right on top of gloves. You're just asking for a rip. You need to make another rubber ring
(butt seal with cyanoacrylate, not overlapped). This time I used 0.06" butyl, to go over the gloves before the clamp is tightly applied.
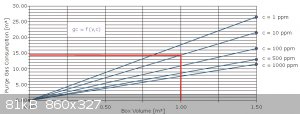
Gas escapes from the glovebox through an oil filled bubbler, and I've come to purge with about 100 ft.³ of gas before use. According to the purge
chart, it's not going to get much better. My vol. is a small fraction of a cubic meter. Let's see, it's about 3 cubic feet, 1 X 1 X 3,... so I guess
it's 20 gal?...whatever... it's only 1/9 of a cubic yard, so less than 0.1 cubic meter. It get's purged with over 30 times its vol to start, and
during several hours work, probably another 30 to 60 vols of gas. The lines were reconfigured to bring argon in at the bottom and exit at top for
obvious reasons.
My gloves are quite old, ridiculously so, but they don't leak. They are 30 mil. I've developed the perfect seal for leaking gloves. it's a combination
of cyanoacrylate to fix the hole and plastic tool dip (like from Home Depot).
[Edited on 23-1-2015 by Dan Vizine]
[Edited on 23-1-2015 by Dan Vizine]
"All Your Children Are Poor Unfortunate Victims of Lies You Believe, a Plague Upon Your Ignorance that Keeps the Youth from the Truth They
Deserve"...F. Zappa
|
|
|
Praxichys
International Hazard
    
Posts: 1063
Registered: 31-7-2013
Location: Detroit, Michigan, USA
Member Is Offline
Mood: Coprecipitated
|
|
Aha
Hey, check this out:
http://pubs.acs.org/doi/abs/10.1021/am900206e
Outgassing of Oxygen from Polycarbonate
Sung In Moon , L. Monson and C. W. Extrand *
Entegris, Inc., 3500 Lyman Boulevard, Chaska, Minnesota 55318
It looks like polycarbonate is susceptible to permeation and adsorption by oxygen. Baking while scrubbing may help, or (unfortunately) replacing the
PC with stainless steel or glass sheets.
Nice glovebox, by the way.
[Edited on 23-1-2015 by Praxichys]
|
|
|
Dan Vizine
National Hazard
   
Posts: 628
Registered: 4-4-2014
Location: Tonawanda, New York
Member Is Offline
Mood: High Resistance
|
|
Quote: Originally posted by Bert  |
The systems I helped build had little heated pots of Ti set up with a charge on them, with a charged grid to accelerate stray gas molecules in the
correct direction to hit the Ti, physically STICK into the surface and react there. Yours truly built the power supplies and fabricated the
electronics chassis.
|
Some of the sputtering chambers that I see have a roughing pump, followed by a turbomolecular pump and the final step, a grid through which liquid
helium flows. There's a little red unit about the size of a medium shop-vac that recyles the helium, actually re-condensing it from gas. They also
charge the grid. It's fairly fast, considering the goal and the large size of the chamber. The doors were large enough that the construction material
was 1" thick SS plate. I has truly impressed.
Your vacuum was probably better still. There were no getters used because, after all, it is a sputtering chamber. I think they'd cause
cross-contamination, but I'm only guessing.
"All Your Children Are Poor Unfortunate Victims of Lies You Believe, a Plague Upon Your Ignorance that Keeps the Youth from the Truth They
Deserve"...F. Zappa
|
|
|
| Pages:
1
2
3 |