| Pages:
1
2
3
4
..
7 |
IOC
Harmless

Posts: 5
Registered: 29-7-2006
Member Is Offline
Mood: No Mood
|
|
yes but if 4-mar is the desired result then C10H15NO will not deliver the goods.
Yes you are absolutley right Nicodem an alanine salt is more than likely the end result of this extraction from the said reaction (almost rhymes :-)
Have any dudes had joy with steam extraction of the PPA freebase from the alanine, H2O and PPA Mix?
The result of ZnCl2 route is still not where every one wants to go so how can the synthesis of aminoalcohols by aldol condesation of aminoacids with
aromatic aldehydes actually produce 12g of PPA from an minimal amout of starting material actually work?
I know there was a methyl group in the write up but what mods can be done to make this more reliable?
Has any dude seen.......
Reaction of the copper complex of L-alanine with acetaldehyde and the mechanism of the akabori reaction
Journal Russian Chemical Bulletin
Publisher Springer New York
ISSN 1066-5285 (Print) 1573-9171 (Online)
Subject Chemistry and Materials Science and Russian Library of Science
Issue Volume 18, Number 11 / November, 1969
Category Organic and Biological Chemistry
DOI 10.1007/BF00906512
Pages 2371-2375
Online Date Monday, January 03, 2005
Add to marked items
Add to saved items
Recommend this article
Organic and Biological Chemistry
Reaction of the copper complex of L-alanine with acetaldehyde and the mechanism of the akabori reaction
V. M. Belikov1, S. V. Vitt1, N. I. Kuznetsova1, M. G. Bezrukov1 and M. B. Saporovskaya1
(1) Institute of Heteroorganic Compounds, Academy of Sciences of the USSR, USSR
Received: 29 July 1968
Conclusions 1. The Akabori reaction involves a stage in which an intermediate imine copper complex is formed from acetaldehyde and copper
alaninate.
2. The intermediate complex has a higher CH acidity than the initial alanine copper complex.
Translated from Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, No. 11, pp. 2536–2541, November, 1969.
no % result given but that acidity could be a key.............
IOC .........out 
|
|
|
IOC
Harmless

Posts: 5
Registered: 29-7-2006
Member Is Offline
Mood: No Mood
|
|
yes but if 4-mar is the desired result then C10H15NO will not deliver the goods.
Yes you are absolutley right Nicodem an alanine salt is more than likely the end result of this extraction from the said reaction (almost rhymes :-)
Have any dudes had joy with steam extraction of the PPA freebase from the alanine, H2O and PPA Mix?
The result of ZnCl2 route is still not where every one wants to go so how can the synthesis of aminoalcohols by aldol condesation of aminoacids with
aromatic aldehydes actually produce 12g of PPA from an minimal amout of starting material actually work?
I know there was a methyl group in the write up but what mods can be done to make this more reliable?
Has any dude seen.......
Reaction of the copper complex of L-alanine with acetaldehyde and the mechanism of the akabori reaction
Journal Russian Chemical Bulletin
Publisher Springer New York
ISSN 1066-5285 (Print) 1573-9171 (Online)
Subject Chemistry and Materials Science and Russian Library of Science
Issue Volume 18, Number 11 / November, 1969
Category Organic and Biological Chemistry
DOI 10.1007/BF00906512
Pages 2371-2375
Online Date Monday, January 03, 2005
Add to marked items
Add to saved items
Recommend this article
Organic and Biological Chemistry
Reaction of the copper complex of L-alanine with acetaldehyde and the mechanism of the akabori reaction
V. M. Belikov1, S. V. Vitt1, N. I. Kuznetsova1, M. G. Bezrukov1 and M. B. Saporovskaya1
(1) Institute of Heteroorganic Compounds, Academy of Sciences of the USSR, USSR
Received: 29 July 1968
Conclusions 1. The Akabori reaction involves a stage in which an intermediate imine copper complex is formed from acetaldehyde and copper
alaninate.
2. The intermediate complex has a higher CH acidity than the initial alanine copper complex.
Translated from Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, No. 11, pp. 2536–2541, November, 1969.
no % result given but that acidity could be a key.............
IOC .........out
|
|
|
IOC
Harmless

Posts: 5
Registered: 29-7-2006
Member Is Offline
Mood: No Mood
|
|
yes but if 4-mar is the desired result then C10H15NO will not deliver the goods.
Yes you are absolutley right Nicodem an alanine salt is more than likely the end result of this extraction from the said reaction (almost rhymes :-)
Have any dudes had joy with steam extraction of the PPA freebase from the alanine, H2O and PPA Mix?
The result of ZnCl2 route is still not where every one wants to go so how can the synthesis of aminoalcohols by aldol condesation of aminoacids with
aromatic aldehydes actually produce 12g of PPA from an minimal amout of starting material actually work?
I know there was a methyl group in the write up but what mods can be done to make this more reliable?
Has any dude seen.......
Reaction of the copper complex of L-alanine with acetaldehyde and the mechanism of the akabori reaction
Journal Russian Chemical Bulletin
Publisher Springer New York
ISSN 1066-5285 (Print) 1573-9171 (Online)
Subject Chemistry and Materials Science and Russian Library of Science
Issue Volume 18, Number 11 / November, 1969
Category Organic and Biological Chemistry
DOI 10.1007/BF00906512
Pages 2371-2375
Online Date Monday, January 03, 2005
Add to marked items
Add to saved items
Recommend this article
Organic and Biological Chemistry
Reaction of the copper complex of L-alanine with acetaldehyde and the mechanism of the akabori reaction
V. M. Belikov1, S. V. Vitt1, N. I. Kuznetsova1, M. G. Bezrukov1 and M. B. Saporovskaya1
(1) Institute of Heteroorganic Compounds, Academy of Sciences of the USSR, USSR
Received: 29 July 1968
Conclusions 1. The Akabori reaction involves a stage in which an intermediate imine copper complex is formed from acetaldehyde and copper
alaninate.
2. The intermediate complex has a higher CH acidity than the initial alanine copper complex.
Translated from Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, No. 11, pp. 2536–2541, November, 1969.
no % result given but that acidity could be a key.............
IOC .........out

|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
2bob, I don't think I understood what you’re asking and saying, but in order to avoid similar questions I figured out it would be quite appropriate
and very polite to the readers, to have the reaction scheme depicted somewhere in this thread (it should fit besides so many nice pictures). A
reaction scheme including the mechanism proposal would be an even better idea to facilitate comprehension.
So here it is:
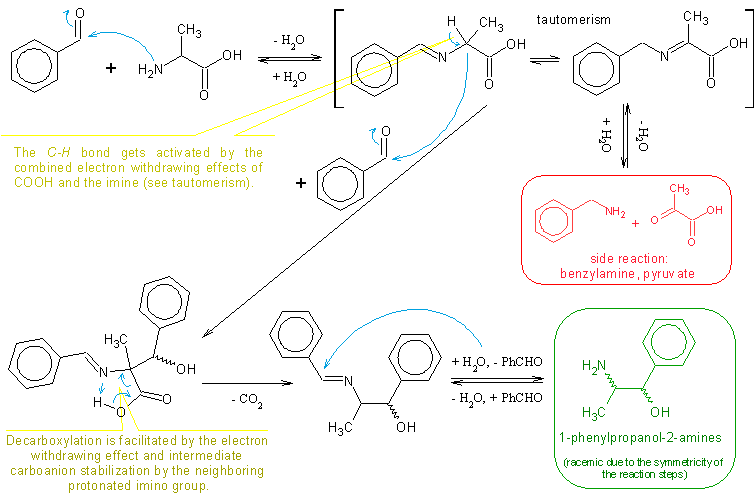
(some obvious steps like H2O eliminations/additions in the imine formation/hydrolysis, proton transfers, carboanion intermediates, resonance
structures and similar are omitted for simplicity sake)
All in all, I think CycloKnight’s latest procedure is quite good even though the yield is only 21%. But then again, the reaction in itself is low
yielding anyway so that yield is not bad at all. It is also worth mentioning that some of the benzaldehyde can be recycled as well as some alanine if
one is ready to purify them.
My compliments to CycloKnight. 
Erratum:
Posted 4 posts above by myself, this sentence:
"Then he would not have to extract the water phase 5 times and still get so little product (assuming the conversion was better than what it looks
like)."
originated from a misreading of the work up description in the CycloKnight’s first post and should be ignored. What I saw as an extraction was
actually just a washing of the extract.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
2bob
Harmless

Posts: 21
Registered: 29-8-2006
Member Is Offline
Mood: No Mood
|
|
Nicotinamide,
I see, but, wouldn't the aldehyde with the CH2 be more effective? (by the look of the structure it would be in the right place). In fact I am about to
try this out, by the look of it, a streckert reduction on L-Ph.alanine => Ph.acetylaldehyde then add l-alanine may yeild l-eph (according to the
rather sparse refs). I personally would prefer l-ps.eph to P2P any day.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
2bob, you are lucky that I'm bored and pissed enough or otherwise I would reply in a very different way. The lucky part is that I’m not bored and
pissed because of you, so you don’t have to take anything personally.
I’m sorry to say this, but you simply don’t SEE.
If you would see, you would see that my nickname is not Nicotinamide and that there is no such member here.
If you would see, you would see that your question makes little or no sense in the context of what you say you can see in my post above.
If you would see, you would see that this is not an illegal drugs and precursors forum and that members here generally don’t use weird and
incomprehensible abbreviations and insinuations.
There are many other things you don’t see, but since I’m in a good mood I will rather give you a link to follow if you ever really want to SEE: https://sciencemadness.org/talk/viewthread.php?tid=6534
I’ll make an exception and will (for the first and hopefully last time) do my best to decrypt your post and thus be able to answer your questions.
First, I need to know what the hell all those abbreviations are. Correct me if I’m wrong but I would estimate this way:
L-Ph.alanine = L-phenylalanine
Ph.acetylaldehyde = 2-phenylethanal
l-alanine = L-alanine
l-eph = L-ephedrine
l-ps.eph = L-pseudoephedrine
P2P = 1-phenylpropan-2-one
“streckert reduction” obviously stands for Strecker degradation
“(according to the rather sparse refs)” must stand for Internet rumors or urban myths
Forgive me if I will have to make some other assumptions. My first such assumption is that “the aldehyde with the CH2” stands for 2-phenylethanal.
Now, I don’t know what you mean by this “[being] more effective”. I would assume you don’t speak about reaction yields, since even assuming
the reaction would work, it would give a totally different product that I tend to believe is of little interest to anybody I know.
So I figured out, that you actually believe 2-phenylethanal would mysteriously give L-ephedrine. This also shows you had no insight in the reaction
scheme (yeah, I know, it is so complex even if you do try).
What you failed to see is that by using 2-phenylethanal and L-alanine you can utmost obtain 3-amino-1-phenylbutan-2-ol (again, assuming the reaction
would work on 2-phenylethanal). Furthermore since the reaction is symmetric at the carbonyl addition point and the only chiral centre on L-alanine is
necessarily racemized in the process (as seen from the reaction mechanism), you can only obtain the racemic mixture of enantiomers. Utmost you could
get an excess of one diastereoisomere over the other.
Now, if 3-amino-1-phenylbutan-2-ol is your goal than go ahead and bleach your “L-Ph.alanine”, but if it is not take some more time and effort to
SEE.
OK, now I feel much better. Have a nice day. 
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
2bob
Harmless

Posts: 21
Registered: 29-8-2006
Member Is Offline
Mood: No Mood
|
|
What a lovely reply,
OK.
Here is the ‘PROPERLY’ expressed argument:
1. I take it that you don’t have an issue that Phenylacetylaldehyde is in fact the major ‘strecker oxidation’ product of phenylalanine?
http://www.dfal.de/EJahr2001.html#1.1.4.
http://pubs.acs.org/cgi-bin/abstract.cgi/jafcau/2005/53/i26/...
The latter publication suggests that not only lipid based oxidants are effective in performing this ‘strecker oxidation’ but also ‘reducing
sugars’.
2. In precisely the same manner that benzaldehyde is the ‘strecker oxidation’ product of toloune?
http://designer-drugs.com/pte/12.162.180.114/dcd/chemistry/b...
3. I also take it as fact, that if the ‘akabori’ reaction between an amino acid and an aldehyde (such as is produced by the ‘strecker
oxidations’ outlined above) is that which is produced in this reaction, thereby validating the reaction, with the important qualification being that
the reaction between benzaldehyde and l-alanine gives phenylpropanolamine and carbon dioxide?
4. Therefore, in order for the researchers to have actually synthesized l-ephedrine/l-pseudoephedrine using the ‘akabori reaction’, as suggested
here:
http://designer-drugs.com/pte/12.162.180.114/dcd/chemistry/e...
Toward the end of the article is a paragraph which states:
‘According to Akabori and Momotani (269), a mixture of an aromatic aldehyde and an amino acid on heating yield alkamines. By means of this reaction,
ephedrine and norephedrine were synthesized.’
They must have taken notice of the loss of the carbon atom (as CO2), and substituted an aldehyde with an additional carbon in order to make the
reaction work (this is my hypothesis, ie. It is a theory, not a fact).
5. However, I see no reason why this would result in the side reaction you claim, otherwise the reaction detailed here would probably have resulted in
an abject failure (although I suggest that yields may be improved by keeping the heat down).
http://www.erowid.org/archive/rhodium/chemistry/nor-pseudo-e...
6. I do however, not really expect that the chirality of the molecule would remain intact throughout, however, I see no reason why I shouldn’t see
if it does, especially as I intend to check to see if it has by use of mandelic acid (prepared by hydrolysis of amygdalin with hydrochloric acid).
Finally, I apologise wholeheartedly for offending your delicate sensibilities by overuse of shorthand for chemical names, and I do sincerely state
that I will endeavour not to reoffend. Additionally, I understand that you would appear to prefer that people didn’t attempt to ‘experiment’
with analogues of previously tried reactions, however, that is the major reason why I have an interest in this form of chemistry, therefore, I make no
apologies for my experimentation.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Great, that is a consolation. After posting, I got a bad feeling I unjustly accused you of “not seeing” while it could have been the consequence
of some disability. I was already afraid I would have to have bad conscience for maltreating a disabled person, but you demonstrated that you
can do better. Thank you.
Now all you have to do is to learn some basic organic and experimental chemistry, learn to follow and read scientific references, see where you have
it all wrong, and way you go!
Finally! A simple and fully OTC route to 3-amino-1-phenylbutan-2-ol!
|
|
|
IOC
Harmless

Posts: 5
Registered: 29-7-2006
Member Is Offline
Mood: No Mood
|
|
My humble, humble apoligies nicoteen or what ever else your calling yourself these days in between alcoholic drinks and fags of cause as it appears
that the edit function isnt an option that is available at the mo, other wise I'd of used it!!!!!!
That function might not be available to me because I also cant spell or have bad grammar or cause Ive got my theories a bit bung or maybe my I's arn't
in a line.....
But I'm sure glad I wasnt that little brat that dobbed other kids in or felt a drunkin urge to correct other contributors about there input to what
ever.......get a life bro
This from memory even if it is short is a thread that CycloKnight started and although you have some pretty drawings to contribute he is really the
only person who has actually had the real experience to talk about
2bob had a valid point in between spelling mistakes, but i guess you'd rather focus on reposts and re filling your drink huh, nice one.......go pour
another one
IOC
|
|
|
IPN
Hazard to Others
  
Posts: 156
Registered: 31-5-2003
Location: Finland
Member Is Offline
Mood: oxidized
|
|
I think a nice picture of some structures should clear this confusion.
http://koti.mbnet.fi/otto2000/products.gif
There is the Akabori reaction product with benzaldehyde and L-alanine, phenylethanal and L-alanine and the product 2bob would like to get with the
Akabori reaction, L-pseudoephedrine.
The difference is quite easily seen...
|
|
|
2bob
Harmless

Posts: 21
Registered: 29-8-2006
Member Is Offline
Mood: No Mood
|
|
seen it, sorry nicodem, but you can at least see what I was hoping for? Thank you for the pictures IPN, I had not noted that, sorry once more.
|
|
|
2bob
Harmless

Posts: 21
Registered: 29-8-2006
Member Is Offline
Mood: No Mood
|
|
now,
at the lower temps, what other amines were formed? Are they still the ephedrine derivatives? I do like it as a possible back road to P2P, although I
know nothing, and must not be allowed to learn in peace, apparently?
|
|
|
kafka
Harmless

Posts: 34
Registered: 26-7-2006
Member Is Offline
Mood: No Mood
|
|
any updates on this project?
|
|
|
Hilski
Hazard to Others
  
Posts: 197
Registered: 13-9-2006
Member Is Offline
Mood: No Mood
|
|
Stirrer/Hotplate Question
This may be a dumb question, but how important is it that the alanine/benzaldehyde be continuously stirred throught the reaction? I only ask this
because I have a mag stirrer, and I have a hot plate, but I don't have a hot plate/stirrer combo.
If it is absolutely required for the sucess of this reaction, then I suppose I'll have to get one when I can afford it.
Thanks.
[Edited on 28-9-2006 by Hilski]
|
|
|
Maja
Hazard to Others
  
Posts: 143
Registered: 27-2-2006
Member Is Offline
Mood: No Mood
|
|
I have tried twice this reaction increasing time even 2x without stirring and yields were at least 2x lower than with stirring... You can try with low
amount to see if it worth your materials and time ...
|
|
|
Hilski
Hazard to Others
  
Posts: 197
Registered: 13-9-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | | I have tried twice this reaction increasing time even 2x without stirring and yields were at least 2x lower than with stirring... You can try with low
amount to see if it worth your materials and time ... |
I may try to make one of these. I suppose it would be better than nothing.
http://www.erowid.org/archive/rhodium/chemistry/equipment/ov...
|
|
|
MARXYZ
Harmless

Posts: 5
Registered: 30-12-2003
Member Is Offline
Mood: Justice. not Corruption
|
|
It was my understanding that Akabori said the reaction resulted in pseudo and/or ephedrine when n-methyl alainine was used. When a primary amine
(alanine) was used, the amine reacted with the aldehyde to form an imine, and did not increase the length of the carbon chain beyond the aldehyde.
Perhapse this is why yields have been low. I would like to see results of n-methyl alanine with an aldehyde. I am also curious what would be the
results of an aldehyde with the calcium salt of pyruvic acid. Also, could N-methyl alanine be synthesized the by reductive amination of the calcium
salt of pyruvic acid with methylamine?
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
N-methylalanine with benzaldehyde gives a mixture of the ephedrines, alanine with benzaldehyde gives a mixture of the norephedrines, as simple as
that. The chirality of the alanines is irrelevant. Check the original papers for other details. The reaction with alanine works just like CycloKnight
described. The yields however vary a lot and you might not be able to reproduce his 21% yield in the first attempt. Stirring is important since the
reaction mixture gets very viscous and will not mix well by itself. Also, lots of CO2 evolves which results in foaming, which I imagine, would carry
the mixture on the top of the condenser if not tempered with efficient stirring.
|
|
|
MARXYZ
Harmless

Posts: 5
Registered: 30-12-2003
Member Is Offline
Mood: Justice. not Corruption
|
|
The problem is that the primary amine forms substantially the side reaction in which the nitrogen bonds directly with the carbonyl to form an imine,
and not the norephedrine. The secondary amine does not take this detour, allowing the carbon to bond with the aldehyde. This is clearly stated in
his named reaction.
|
|
|
jon
Hazard to Others
  
Posts: 459
Registered: 11-1-2006
Member Is Offline
Mood: paranoid distrustful apprehensive
|
|
man in that mechanism there is the tautomerizm you were talking about and the main product of the reaction is bis-phenyl-2-aminoethan-1-ol. I'm not
sure how that's formed but I this perhaps stoichiometry is the reason that the first reaction went off so well as the aldehyde was added slowly. then
temperature could make the tautomer more stable in one position rather than the other. I know that tautomer is the reason the principal byproduct is
formed in the case of alanine but not in the case of the secondary amine (because of enamine formation as opposed to a tautomeric imine)
In the original reference I noticed that pyridine was used in the reaction with alanine to get 17% and without it it's a crapshoot I think pyridine
serves as a base and changes the equilibrium between the two tautomers .temperature (165 celcius in the first reaction) may do the same thing.
another thing the bis compund I'm talking about is that lower phase you see in the seperation it's hydrochloride is insoluble in water as you see in
the photo the bottom layer of the three.
and as for extraction the way to do this is too make the aqueous
alkaline, saturate with salt and then add isopropanol. the isopropanol will seperate clean and take the amines with it leaving the salts in the
aqueous the isopropanol is then evaporated. that's how ppa is worked up.
oh another thing you think the alanine in the absence of water will protonate the shiff's base of the ppa produced? and affect it's solubility in the
organic solvent used to extract it with? that's what your'e actually extracting and that's why half of the benzaldehyde does'nt react any further it's
associated with the end product as a shiff's base.
ok so after you extracted the amines into toulene and let's assume you have some amines that are associated with the amino acid sludge that's left
over how would you get at them? treat with base, and then what? you'd have a mess of alanine salts seems like a pain in the arse.
[Edited on 3-10-2006 by jon]
[Edited on 3-10-2006 by jon]
|
|
|
ONDDOLE
Harmless

Posts: 1
Registered: 9-11-2006
Member Is Offline
Mood: No Mood
|
|
Some help plz?
In regards to cycloknights 2nd PPA synth after washing the aqueous amine solution with some dilute HCl solution, swih picture shows pink solution in
the flask...
Problem- swim has given it ago however upon wash(HCl 10%=volume) swims aqueous solution is yellow?
any ideas why? swim used xylene instead of toleune could this be the reason?
also washing with DCM could just abit of acetone be used instead?
|
|
|
jon
Hazard to Others
  
Posts: 459
Registered: 11-1-2006
Member Is Offline
Mood: paranoid distrustful apprehensive
|
|
wash the aqueous phase with acetone? you can try it but I'll think you'll be dissapointed.
acetone is miscible with water.
I think it was mentioned that this reaction has a degree of irreproducibility sometimes you'll get varied yeilds and so a different product
composition can be expected and hence the color of the HCl extract.
|
|
|
haribo
Harmless

Posts: 24
Registered: 28-11-2006
Member Is Offline
Mood: No Mood
|
|
Just out of interest, do you end up with a raecemic mixture? I ask because the cyanate route to 4MAR (the one that's 1-pot and uses safe chemicals!)
only works on trans isomers (I think). Of course, if you lose a carbon by using glycine then you don't have that problem.
EDIT by The Davster:
Keep it chemistry, not how well it smokes and its effects. That has been deleted.
[Edited on 30-11-2006 by The_Davster]
|
|
|
haribo
Harmless

Posts: 24
Registered: 28-11-2006
Member Is Offline
Mood: No Mood
|
|
Sorry about that. Yes, I'm interested in knowing if anyone has tried this particular reaction replacing the alanine with glycine. The product has only
1 optical centre and therefore, should undergo cyclization to an oxaziline ring using the enviroment (and chemist) friendly cyanate route. Even if the
yield is only 20%, if cyclization can be performed with 78% yield (or their abouts) you would still end up with 17% product. Not GREAT, I agree, but
for the availability/cost of the precursors, it really would be cheap.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Given that the resulting compound would be 1-phenyl-2-aminoethanol, I doubt anybody would consider wasting time and resources with an Akabori reaction
when the same compound is easily accessible from styrene in two simple and well documented steps both having yields above 70%.
|
|
|
| Pages:
1
2
3
4
..
7 |