franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Hypothetical Molecules
Everyone at times imagines interesting assemblies of atoms and the
molecules that might be made. This superb thread by Axt ->
http://www.sciencemadness.org/talk/viewthread.php?tid=4282#p...
reminded me of an idea I had. EDNA being acid ( pg 394 Davis ) can
condense with metal hydroxides forming their salt. Aluminum in particular
it appears will form a nicely compact molecule. Two moles Al to three
of EDNA will give Al2[C2H4(NNO2)2]3 ( I'm not a chemist stop laughing ) 
well anyway this is it structurally _

This does not convey its 3 dimensional shape so I made some models using
Arguslab / free download here -> http://www.arguslab.com
In all of these the two metal atoms are seen in the center of the cluster.
Nitrogen appears Blue , Oxygen is Red , Carbon shows as Grey ,
with Hydrogen in White.

C L I C K F O R F U L L S I Z E
http://img28.imagevenue.com/img.php?loc=loc161&image=115...
Upon disarrangement it becomes -> Al2O3 + 3CO2 + 3CO + 6N2 + 6H2
a total of 19 moles ! This assures it will be brisant. It is somewhat
oxygen deficient however. If three methylene groups could be left out
then this would be stoichiometric. This baits the question what if one
could do this same thing using methylene dinitramine CH2(HNNO2)2 ?
Well I know there is methyl nitramine so this is not a stretch.
Structurally it would be _ Al2[CH2(NNO2)2]3


C L I C K F O R F U L L S I Z E
http://img27.imagevenue.com/img.php?loc=loc138&image=115...
Upon disarrangement it becomes -> Al2O3 + 3CO2 + 3H2O + 6N2
a total of 13 moles and oxygen balanced !
A further modification that suggests itself would be to have
instead of the Aluminum , two atoms of Silicon joined by a
single bond down the center of the molecule. Si2[CH2(NNO2)2]3

C L I C K F O R F U L L S I Z E
http://img101.imagevenue.com/img.php?loc=loc33&image=115...
disarranged it becomes 2SiO2 + 3CO2 + 2H2O + 6N2 + H2
a total of 14 moles and almost oxygen balanced. But oxidizing
two silicon bonds is better than two of hydrogen.
.
[Edited on 21-6-2006 by franklyn]
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Metal Fluoro Hydrazides
If you want the highest attainable energy you are very much
limited to reactions involving metals with oxygen or flourine.
In keeping with my post here I think it possible to produce a
molecule that meets this criteria and is still stable and not
particularly sensitive.
If one begins with Difluorohydrazine and sequentially reacts this
with metal hydroxides, a rather compact molecule can be formed, thus
3H2NNF2 + Al(OH)3 -> Al(HNNF2)3 + 3H2O
2Al(HNNF2)3 + 3Mg(OH)2 -> Al2Mg3(NNF2)6 + 6H2O
So we have the hypothetical
DiAluminum Trimagnesium Hexadifluorhydrazide

C L I C K F O R F U L L S I Z E
http://img143.imagevenue.com/img.php?image=24058_DiAluminumT...
Al2Mg3(NNF2)6 -> 2AlF3 + 3MgF2 + 6N2
This has high energy high density and detonated evolves 11 moles
I would even venture to guess it's VOD would exeed 9 Kps.
Any Crticisms ?
.
[Edited on 28-7-2006 by franklyn]
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
Oh so the proton attaches to an oxygen of the nitroanmine group
. . . .N = O
. . . . |
H . . N - O - H
. \ . /
. .C
. / . \
H . . .N - O - H
. . . . .|
. . . . .N = O
http://www.sciencemadness.org/talk/viewthread.php?tid=3033
So why didn't somebody say something 
Not so compact a structure then afterall.
.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
The "bond" between cations and anions is not a bond, it is only a ionic interaction. Hence there exist no such things as the molecules you draw. These
salts would have crystal structure.
Many bonds between metals and nonmetals can indeed be considerably covalent, but as a rule of thumb: if the conjugated acid of the anion part has a
low enough pKa (= is electonegative enough) even Ag will not form a covalent enough bond to allow speaking about any kind of molecules. So you can be
sure Al, Mg or other such highly electropositive metals will not form them with the relatively acidic nitroamines.
You should really read on basic chemistry, especially notice the difference between anorganic and organic chemistry, ionic and covalent interactions
and so on. (meant as an sincere advice to take or leave, after all in elementary school I did the same exact mistakes  ) )
Edit: I just realized that "rule of thumb" I made up is not useful as the polarization properties of the anion have even more influence on the
ionic/covalent character of the bond. For example the conjugated acids of sulfates and iodides have approximately the same pKa and yet AgI is mainly
covalent while Ag2SO4 is mainly ionic (iodide is highly polarasible, sulfate is not). Similarly HgO is way more ionic than HgS even though H2S is more
acidic than H2O and many similar examples. Sorry for the missinformation.
[Edited on 30-7-2006 by Nicodem]
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
My post here may be speculative but not uninformed
from the library here _
http://www.sciencemadness.org/library/books/the_chemistry_of...
See page 394
The compound MEDINA which I just found out about cannot be much
different in action. Aluminum and Magnesium form carbonates and
that is very weakly acid. Calcium forms Cyanamide CaNCN and it
is even more electropositive.
Your assertions seem cotradictory and only raise more questions.
Now if you critisize my visualizations note that I just realized that
the anion will form with the oxygen. Thus the attachmentwith a
Nitrogen is in error. This may also be true with the hydrazide which
cannot self ionize. If this could not work with hydroxides then
perhaps their chlorides can be reacted. Nitrides of Aluminum and
Magnesium are well known, and attaching other nonmetalic atoms
as functional group is not so far fetched.
__________________________________
U P D A T E
| Quote: | Originally posted by Nicodem
Sorry for the missinformation. |
It's significance is probably lost on me anyway.
I maintain chemistry is no more a science than
zoo-ology or botany in which the science is in
the cataloging of species. I offer as proof of this
the frequent use of the words ' surprising '
and ' unexpected ' appearing in scholarly papers
and treatise on chemical research and investigation.
[Edited on 30-7-2006 by franklyn]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
But what have all those examples and interpretations to do with my mention that the salts you propose can not form molecules as it is implied in the
structures on the pictures?
After all, if they are plainly salts, as even you seam to realize, than it should be pretty obvious they can not be molecules at the same time. So
your claim of their grater density which is based on molecular and not crystal structure is without any ground. I don't know what the densities of
your salts would be. They might be quite high, but the hypothesis on which you base the claim don't apply. I hope someone with knowledge on crystal
chemistry will be able to provide an applicable hypothesis, but that won’t be me as that is not my field and I don’t know much about it (Tim,
could you please?).
Translation in short and simple words:
You should base your claims on crystal structure and everything would be just fine.
I sincerely hope that now you understood what I meant and not misunderstood again. And yes, I know, I should provide a links describing the difference
between ionic and covalent interactions but I'm not in that kind of mood right now.
| Quote: | | I maintain chemistry is no more a science than zoo-ology or botany in which the science is in the cataloging of species. I offer as proof of this the
frequent use of the words ' surprising ' and ' unexpected ' appearing in scholarly papers and treatise. |
I don't see them often anymore. I do however agree that ignorant teachers like to use them a lot, but they don't understand much about mechanisms
underlaying chemical reactions. Furthermore, I noticed that even some chemists who understand the mechanistics tend to be "believers", that is, they
keep forgeting that these are only theories (only cognitive tools). If you see chemsitry similar to "cataloging of species" then you have still a lot
to learn about how matter behaves. Botany and zoology is also not just taxonomy or there would not be any need for this separate term for the
knowledge of systematization.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Axt
National Hazard
   
Posts: 778
Registered: 28-1-2003
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by franklyn
So why didn't somebody say something 
|
Thats not MEDINA, which is a true nitramine -NH-NO2 (or aci form -N=N(O)OH). -N(OH)-NO is an isonitramine, nitrosohydroxylamine, or in recent
literature as diazeniumdiolates refering to its tautomeric form -N<sup>+</sup>(O<sup>-</sup> =NOH. =NOH.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
You both have me as cofused as I hope to be, clearly I am out of my depth here.
______________________
U P D A T E
I do not go to far afield from what is known ,
in surmising if something may be possible.
Again from the library here _
http://www.sciencemadness.org/library/books/the_chemistry_of...
On page 377 in the center of that page is described
Calcium Hydrogen Cyanamide
NH - CN
|
Ca
|
NH - CN
Now if three moles of this can be reacted with two of Al(OH)3
to form a larger arrangement much as what I have graphically
depicted above, then the issue is closed.
It doesn't matter wether the Cyano is instead a Nitro or a
diFluoromide.
As to wether this is materially a unitary molecule or a salt lattice
of ions , I don't know , and that is secondary to wether or not
it can form at all.
I naively assume that the textual representation faithfully
shows the actual arrangement of the constituent atoms.
As for density that is a property of crystal formation and the
mass of its atoms independant of chemical character.
IN
http://www.sciencemadness.org/library/books/the_chemistry_of...
On page 394
this talks about alkali salts of EDNA
IN Vol IV
Urbanski 'Chemistry and Technology of Explosives
On page 363
this talks about corrosive acidity of EDNA on metals
IN Vol III
Urbanski 'Chemistry and Technology of Explosives
On page 17
this talks about MEDINA first isolated from its Barium salt
.
[Edited on 1-8-2006 by franklyn]
|
|
|
AngelEyes
Hazard to Others
  
Posts: 187
Registered: 24-1-2003
Location: South of England
Member Is Offline
Mood: Better than it used to be.
|
|
How about this?
I guess it might be called CycloTriCarbonylTriNitrosAmine (CTCTNA).
Similar to CTMTNA that can be prepared from Hexamine and Nitrous acid...but with the 2 hydrogens on each of the 3 carbons replaced by a double bonded
Oxygen.
Maybe it's not a feasible molecule, someone may well point out some error with bond angles or lengths etc, but I figured this to be the best topic
under which to post it.
On the plus side, it would be completely Oxygen balanced with no water at all in the decomposition products.
Other than trying to selectively oxidise CTMTNA I'm not sure how one might try to prepare it...but hey - this is all hypothetical, right?
I have attached the molcular structure of it I drew using ArgusLab. Can't seem to insert the pic straight into this post though. Ho hum...I'm sure you
could already guess what it looked like anyway.
Cheers
Angel
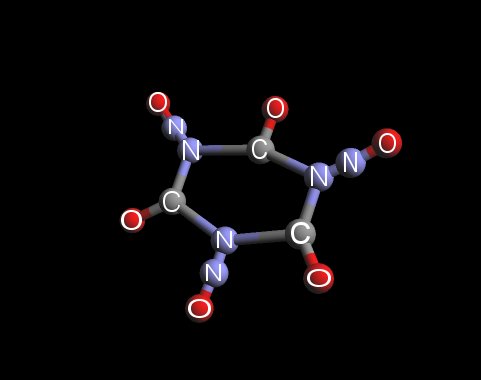
\'Silk and satin, leather and lace...black panties with an Angel\'s face\'
|
|
|
Nick F
Hazard to Others
  
Posts: 439
Registered: 7-9-2002
Member Is Offline
Mood: No Mood
|
|
"I naively assume that the textual representation faithfully shows the actual arrangement of the constituent atoms."
No, it doesn't. It's just more convenient than drawing an infinite 3D lattice on paper!
Those drawings of CaC2 show sp hybridised carbon atoms covalenty bonded with approximately 60 degree bond angles, which just doesn't happen. There's
not enough electron density at that angle to form a bond of any significance.
|
|
|
Nick F
Hazard to Others
  
Posts: 439
Registered: 7-9-2002
Member Is Offline
Mood: No Mood
|
|
Also, lots of people seem to be balancing their ideas to CO2 and H2O, whereas most work seems to be done on molecules balanced to CO and H2O - the
extra volume of gas produced per gram outweighs the fact that the carbon could be further oxidised.
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by AngelEyes
I guess it might be called CycloTriCarbonylTriNitrosAmine (CTCTNA).
Similar to CTMTNA that can be prepared from Hexamine and Nitrous acid...but with the 2 hydrogens on each of the 3 carbons replaced by a double bonded
Oxygen.
[...]
On the plus side, it would be completely Oxygen balanced with no water at all in the decomposition products.
Other than trying to selectively oxidise CTMTNA I'm not sure how one might try to prepare it...but hey - this is all hypothetical, right?
[...]
|
That would be more appropriately called by using trivial names, that is trinitrosoisocyanuric acid. Theoretically it might be possible to prepare it
by nitrosating cyanuric acid. As far as what would be the working method of doing this is beyond my knowledge. Simply nitrosating cyanuric acid with
NaNO2 and HCl might eventually work, but I have certain doubts. In the presence of water it might slowly decompose to CO2 and N2. Of course one would
have to check if the compound is not known and described already.
However, the compound does not seam very “energetic” to me. The carbons are already all in the state of their highest oxidation number (+4), so
the only flow of electrons that can happen is from the “amide” nitrogens toward the “nitroso” nitrogens followed by rearrangement (hopefully
ending in an exothermic cascade resulting in an explosion). This would formally give 3 mols CO2 and 3 mols N2 per mol of trinitrosoisocyanuric acid.
However, the low electron density on the “amide” nitrogens would make the activation energy high while the released energy is already low due to
the already mentioned reasons (thus the reaction enthalpy would be even lower than the similar burning of NH4NO2). It might be very hard to detonate
if possible at all. But then again I don’t know much on explosives.
PS: Needless to mention that trinitrosoisocyanuric acid, just like all nitrosoamides, would be a powerful carcinogen.
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Your imagevenue image doesn't seem to be there.
Both borazine and melamine are flat molecules, resonance stabilised. Why would they form out-of-plane bounds that destroy their aromaticity? Doing so
would require shoving energy into the reaction to create the proposed product, note that the documented chemistry of borazine almost entirely gives
either flat graphite-like structures, addition with preservation of the existing bonds, or the fragmentation of the ring. Much of melamine's reactions
are those of the amino hydrogens, not the ring bonds.
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Nick F
lots of people seem to be balancing their ideas to CO2 and H2O, whereas most work seems to be done
on molecules balanced to CO and H2O - the extra volume of gas produced per gram outweighs the fact that
the carbon could be further oxidised. |
Yes I'm aware of that, it derives from the gas laws. The best example
of this
is equal weight comparison of Hexanitrosobenzene and Hexanitrobenzene, these
are quite similar in output despite their individual stoichiometry. The presence
of a high percentage of uncombusted Hydrogen yeilds an even better effect
particularly with high energy slurry mixtures. It would be of interest to see a
formalized investigation of this outlining the optimal theoretical oxygen balance
which I'm sure has been done many times by specialists in this field.
[Edited on 30-8-2006 by franklyn]
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by franklyn
EDNA being acid ( pg 394 Davis ) can condense with metal hydroxides forming their salt.
Can Aluminum in particular do this same thing using methylene dinitramine CH2(HNNO2)2 ?
Structurally it would be _ Al2[CH2(NNO2)2]3 |
http://www.sciencemadness.org/talk/viewthread.php?tid=3033#p...
.
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Note that the aluminium is already oxidised to the 3+ state in such compounds, so there's little energy available from it. That's just another salt,
like Al2(SO4)3.
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
What if you take an organo-aluminum compound and crosslink the substituents, possibly shielding it (er... Al(tBu)3 or so?) and adding more reactive
groups, nitro- for example?
Tim
|
|
|
franklyn
International Hazard
    
Posts: 3026
Registered: 30-5-2006
Location: Da Big Apple
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by not_important
Note that the aluminium is already oxidised to the 3+ state in such compounds, so there's little energy available from it. That's just another salt,
like Al2(SO4)3. |
Thats the best argument against I've heard yet. In my zeal I overlooked the oxidation
potential. Still there would be some gain going to a flouride for the other ' salt '.
[Edited on 4-9-2006 by franklyn]
|
|
|