| Pages:
1
2 |
pantone159
National Hazard
   
Posts: 586
Registered: 27-6-2006
Location: Austin, TX, USA
Member Is Offline
Mood: desperate for shade
|
|
ketone-bisulfite product recrystallization
I've heard that ketones form addition products with bisulfite, which are crystalline and so should be purifyable by recrystallization.
I just tried this using acetone as the ketone. I dissolved about 25 mmol of Na metabisulfite (=50 mmol Na bisulfite) in water, diluted this with some
EtOH, and added 50 mmol of acetone. At first, everything dissolved, but soon I got a cake of white crystals which I assume is this addition product.
What are good recrystallization solvents for this, and other ketone-bisulfite products? I'll try some things but it would be nice if anybody has any
hints.
Do these compounds have names?
|
|
|
nuclear
Harmless

Posts: 17
Registered: 10-2-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by pantone159
Do these compounds have names? |
This compounds are hydroxysulphonates.
Recrystallize it from hot water (?), then add dilute acid or base and distil mixture.
[Edited on 30-6-2006 by nuclear]
|
|
|
Baphomet
Hazard to Others
  
Posts: 211
Registered: 19-11-2006
Member Is Offline
Mood: No Mood
|
|
So Na metabisulfite works in a similar way to it's 'non-meta' counterpart? (I was under the impression it didn't). Have you confirmed that the adduct
was formed successfully?
BTW you can wash with water to clean it but the bisulfite adducts are not water soluble so another solvent would be better for recrystallisation
\"Who ARE you? You\'re like the drummer from REO Speedwagon - nobody knows who you are\" from \'Employee of the Month\'
|
|
|
chemrox
International Hazard
    
Posts: 2961
Registered: 18-1-2007
Location: UTM
Member Is Offline
Mood: LaGrangian
|
|
Making the addition product is the purification since only the carbonyl compound undergoes the reax. You then collect the precipitate and react it
back to normal state. Then you have to dry it or whatver is indicated .. often distillation. You don't recrystallize the addition product.
[Edited on 18-2-2007 by chemrox]
[Edited on 18-2-2007 by chemrox]
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
chemrox is correct, usually the addition compound is formed, collected, washed with a bit of cold bisulfite solution, and then decomposed with acid or
base to free the carbonyl compound which is extrated from the aqueous products.
The addition compound does have some solubility in water. This can be used when you are removing small amounts of a carbonyl compound that is either
left over after a reaction or formed as a side product. the organic layer is washed several times with not too concentrated bisulfite, which removes
the carbonyl. For a few of the addition compounds the solubility is high enough that it is left in aqueous solution, which is washed with an organic
solvent to removed non-carbonyl compounds.
The following thread is a recent discussion that included a bit on the bisulfite addition. I posted a short list showing the reactivity of various
carbonyl compounds to bisulfite, which shows some ofthe limitations of the reaction.
http://www.sciencemadness.org/talk/viewthread.php?tid=7689
|
|
|
pantone159
National Hazard
   
Posts: 586
Registered: 27-6-2006
Location: Austin, TX, USA
Member Is Offline
Mood: desperate for shade
|
|
No good recrystallization
I never posted this result (maybe should have), but I did try and recrystallize the acetone-bisulfite complex with various solvents (would have to
look up details) and nothing worked. My conclusion was indeed, not to bother, nothing could be accomplished.
|
|
|
Sergei_Eisenstein
Hazard to Others
  
Posts: 290
Registered: 13-12-2004
Location: Waziristan
Member Is Offline
Mood: training
|
|
It's not a bad idea to wash the adduct with ether.
damnant quod non intelligunt
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
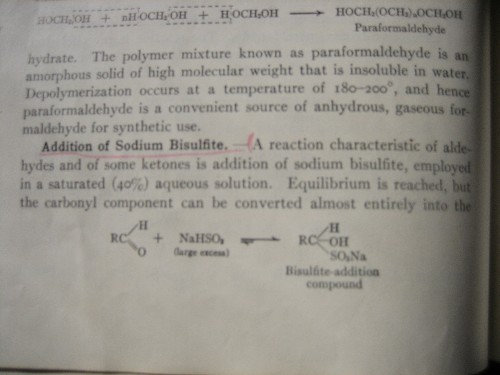
For those wishing a little more detail on what the bisulfite adduct looks like for an aldehyde, below is an excerpt from Introduction to Organic
Chemistry by Fieser & Fieser, 1957, p. 158.
Someone challenged the use of sodium metabisulfite for this application vs sodium bisulfite. It has always been my impression that sodium
metabisulfite, Na2S2O5, converts to NaHSO3 in aqueous solution, i.e.,
Na2S2O5 + H2O --> 2NaHSO3
Isn't this true?
[Edited on 18-2-2007 by Magpie]
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|
pantone159
National Hazard
   
Posts: 586
Registered: 27-6-2006
Location: Austin, TX, USA
Member Is Offline
Mood: desperate for shade
|
|
| Quote: | Originally posted by Magpie
It has always been my impression that sodium metabisulfite, Na2S2O5, converts to NaHSO3 in aqueous solution, i.e.,
Na2S2O5 + H2O --> 2NaHSO3
Isn't this true?
|
Yes, that is right. Also, metabisulfite is more stable, easier to prepare pure (bisulfite will tend to turn into metabisulfite), and usually cheaper.
Metabisulfite is the way to go.
|
|
|
pantone159
National Hazard
   
Posts: 586
Registered: 27-6-2006
Location: Austin, TX, USA
Member Is Offline
Mood: desperate for shade
|
|
| Quote: | Originally posted by Sergei_Eisenstein
It's not a bad idea to wash the adduct with ether. |
Vogel (3rd ed) does suggest this.
|
|
|
Baphomet
Hazard to Others
  
Posts: 211
Registered: 19-11-2006
Member Is Offline
Mood: No Mood
|
|
I stand corrected, meta is the way to go.
Does anyone have experience with releasing the adduct back to freebase ketone?
The article by Eleusis advises that one should extract into a non-polar solvent then vac distill to isolate
But if we're trying to avoid a cumbersome vac setup by using the adduct, doesn't it defeat the purpose?
Would it be possible to simply release the ketone with a basic solution in a sep funnel and obtain the oil that way (assuming of course a ketone that
is not water soluble)?
\"Who ARE you? You\'re like the drummer from REO Speedwagon - nobody knows who you are\" from \'Employee of the Month\'
|
|
|
pantone159
National Hazard
   
Posts: 586
Registered: 27-6-2006
Location: Austin, TX, USA
Member Is Offline
Mood: desperate for shade
|
|
| Quote: | Originally posted by Baphomet
But if we're trying to avoid a cumbersome vac setup by using the adduct, doesn't it defeat the purpose?
|
Another purpose could be so you could store the adduct, while the methyl ketone/aldehyde might go bad quickly. By partially purifying it, you might
make the vac distillation go easier, also. (If your raw material is contaminated with other methyl ketones/aldehydes, they would form an adduct too,
so you still probably need to vac distill to get rid of them.)
I have no experience with actually doing any of this, however.
[Edited on 19-2-2007 by pantone159]
|
|
|
roamingnome
Hazard to Others
  
Posts: 363
Registered: 9-9-2006
Member Is Offline
Mood: No Mood
|
|
how annoying that i cant find the reference paper i went with, but just yesterday i made 20 grams of the benzaldehyde bisulfite adduct.
now all i had was sodium sulphite which had me worried
but the old school paper i found discussed that adding H2SO4 drives the equilibrium to completion by reacting with formed hydroxide and keeping HSO3
as the active species.
having a pH meter on hand allowed me to just keep the pH below 7 with a few drips of acid. the "ester like" adduct was nice to see.
and pure IPA wash was second place from an ether wash....
now to extract this to oil, it would be nice to have 100% capture without distillation(which i normally have no problem with)
from old posts, pouring in hot carbonate base water should release SO2 gas and oil.
i hope to finally wip up a laboratory-butane-extractor. let it evaporate for now and it best leave some oil behind... i hope...
as a non-polar i want to work more with butane, i hope it solvates stuff well..hmmm
[Edited on 19-2-2007 by roamingnome]
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
| Quote: | Originally posted by Baphomet
I stand corrected, meta is the way to go.
Does anyone have experience with releasing the adduct back to freebase ketone? |
'freebase' is an incorrect term in this case, just "free ketone" is better.
| Quote: | The article by Eleusis advises that one should extract into a non-polar solvent then vac distill to isolate
But if we're trying to avoid a cumbersome vac setup by using the adduct, doesn't it defeat the purpose?
Would it be possible to simply release the ketone with a basic solution in a sep funnel and obtain the oil that way (assuming of course a ketone that
is not water soluble)? |
You use a solvent for the same reason you often use a solvent when you're just washing an organic compound. The solvent reduces mechanical loses, may
make it easier to use the sep funnel - larger difference in specific gravities, lower viscosity, other physical attributes may be better with the
solvent. Sometime the desired compound has some solubility in water and using a solvent reduces losses from that.
If the solvent and the product have widely differing boiling points, you might get away without using a vacuum rig. If they do differ greating in
boiling point, but both are fairly high boiling, you might want to use vacuum to reduce the temperature the product is exposed to.
Sometimes you want to distill anyway because there can be sideproducts that are also carbonyl compounds and they have followed the desired product
through the bisulfite cleanup. The main point of the bisulfite method is to remove maerials that don't for the addition product, there can always be
other gunk to worry about.
Note that some carbonyl compounds that have alpha, beta unsaturation can undergo 1,4 addtion with bisulfite. Not real common, but caution should be
take with that sort of compound.
And, yes, the bisulfite addition compound can be useful for storing aldehydes by reducing the oxidation rate. You can also find the bisufite addition
compound with formaldehyde and acetaldehyde as an easier to handle form of those reagents.
|
|
|
roamingnome
Hazard to Others
  
Posts: 363
Registered: 9-9-2006
Member Is Offline
Mood: No Mood
|
|
http://pubs.acs.org/cgi-bin/abstract.cgi/ancham/1947/19/i12/...
use of sodium sulfite and H2SO4
can adding the bisulfite-adduct to a reaction be of use forming acetaldehyde or the like in situ..... or is adding the free aldehyde or ketone group
more reactive and overall better....
|
|
|
jon
Hazard to Others
  
Posts: 459
Registered: 11-1-2006
Member Is Offline
Mood: paranoid distrustful apprehensive
|
|
I've seen where the bisulfite adduct can be formed under phase transfer conditions ie. the use of a phase transfer catalysyt i think the one employed
was tetrabutylammoniumbromide (TBAB) It's useful when you have some really dirty ketone. barring this I've also heard the adduct is insol. in ethanol,
ethanol should do a good job or removing impurities.
the adduct is somewhat sol. in H2O so that's why in most examples a conc. sol. of NaHSO3 is used as a wash.
[Edited on 21-2-2007 by jon]
|
|
|
EmmisonJ
Hazard to Self
 
Posts: 89
Registered: 5-1-2009
Member Is Offline
Mood: No Mood
|
|
sorry to resurrect an old thread
Quote: Originally posted by Magpie  | It has always been my impression that sodium metabisulfite, Na2S2O5, converts to NaHSO3 in aqueous solution, i.e.,
Na2S2O5 + H2O --> 2NaHSO3 |
so if someone wanted to form an adduct using h2o as a medium via this method using metabisulfite then you would scale down the molar ratio by half in
comparison to bisulfite it sounds like?
upon checking water solubility of metabisulfite and bisulfite i'm confused. metabisulfite's water solubility is 54 g/100 ml and bisulfite's water
solubility is 42 g/100 ml. so you would assume since one mole of metabisulfite and one mole of h2o = 2 moles of bisulfite that technically the water
solubility of metabisulfite would be 21 g/100ml since it forms 2 moles of bisulfite right? i don't know enough about it to make sense of it
[Edited on 2-8-2009 by EmmisonJ]
|
|
|
not_important
International Hazard
    
Posts: 3873
Registered: 21-7-2006
Member Is Offline
Mood: No Mood
|
|
Can't forget kinetics and reaction rates. The expected conversion may be slow, especially if the bisulfite isn't being removed by reaction with
something.
|
|
|
EmmisonJ
Hazard to Self
 
Posts: 89
Registered: 5-1-2009
Member Is Offline
Mood: No Mood
|
|
would it be possible to use a sodium bisulfite solution to recover ketones/aldehydes from a nonpolar solvent such as ether, toluene, etc? i read that
bisulfite isn't very soluble in ethanol and i'd imagine that the sodium bisulfite must be made soluble in the nonpolar solvent in order to react with
the ketone/aldehyde, if so then i guess one would use a satured sodium bisulfite solution then add enough alcohol to make the solution more soluble in
the nonpolar solvent (ie: 70% or more of methanol in relation to water used to dissolve the bisulfite) or would that just crash out the sodium
bisulfite due to it not being very soluble in alcohols?
[Edited on 2-8-2009 by EmmisonJ]
[Edited on 2-8-2009 by EmmisonJ]
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Yes, aldehydes (I don't know about ketones, but maybe if they are small and reactive enough) can be extracted into aqueous NaHSO3 of sufficient
concentration. This is however not a particularly popular method of separation (in comparison to the common bisulfite adduct precipitation in EtOH)
due to several reasons.
| Quote: | | i read that bisulfite isn't very soluble in ethanol and i'd imagine that the sodium bisulfite must be made soluble in the nonpolar solvent in order to
react with the ketone/aldehyde |
I don't know about any other bisulfites, but sodium bisulfite is soluble enough in ethanol or slightly diluted ethanol. That is, soluble enough to
react with aldehydes and some ketones - obviously since on this is the classical bisulfite adduct precipitation based on. Not all NaHSO3 needs to be
in solution. If you let it stir enough and/or slightly heat it, it will react even under heterogenous conditions. Just follow any literature example
and don't mind if the bisulfite crashes out after ethanol addition. If, on the other hand, you dilute with too much water (that is, add too little
ethanol) the bisulfite adduct might not precipitate (or only little will), because the solubility of these compounds in water can be very good.
|
|
|
Barium
Hazard to Self
 
Posts: 85
Registered: 24-8-2008
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by EmmisonJ  | | would it be possible to use a sodium bisulfite solution to recover ketones/aldehydes from a nonpolar solvent such as ether, toluene, etc?
|
A aqueous solution of bisulfite/metabisulfite and a PTC (phase transfer catalyst) such as TBAB, Aliquat 336 or similar will do just that.
|
|
|
EmmisonJ
Hazard to Self
 
Posts: 89
Registered: 5-1-2009
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Nicodem  | | I don't know about any other bisulfites, but sodium bisulfite is soluble enough in ethanol or slightly diluted ethanol. That is, soluble enough to
react with aldehydes and some ketones - obviously since on this is the classical bisulfite adduct precipitation based on. Not all NaHSO3 needs to be
in solution. If you let it stir enough and/or slightly heat it, it will react even under heterogenous conditions. Just follow any literature example
and don't mind if the bisulfite crashes out after ethanol addition. If, on the other hand, you dilute with too much water (that is, add too little
ethanol) the bisulfite adduct might not precipitate (or only little will), because the solubility of these compounds in water can be very good.
|
that's a good idea, so i'm guessing how that would work is to oversaturate the EtOH and as it reacts with the aldehyde or ketone and forms the adduct
it will crash out, then more sodium bisulfite will dissolve in its place which will then form more adduct and so on until it all eventually reacts.
increasing heat to help solubility and speed up the process is a great idea.
wouldn't happen to know sodium bisulfite's solubility in EtOH and/or MeOH do you?
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Just follow a literature example and stop complicating. There are literally thousands of examples described in the literature. This used to be a
general method to clean aldehydes before flash chromatography made everybody go on automatic mode.
If you want to learn about the theory of heterogeneous reactions, this is not the best example to discuss, as it does not even need to be
heterogeneous (actually it is not even expected to be such). Aldehyde will form an adduct in matter of seconds even without much NaHSO3 excess, while
ketones need a twofold or more excess of NaHSO3 and much more time, and still the precipitation is not complete (the equilibrium constant for ketones
is nearly not as high as you might hope).
|
|
|
simba
Hazard to Others
  
Posts: 175
Registered: 20-5-2011
Member Is Offline
Mood: No Mood
|
|
I'm a little puzzled by these bisulfite adducts, I tried extracting vanillin from solution last week, and nothing precipitated out of it. I mixed
sodium metabisulfite with cold water, allowed everything to dissolve, added to the vanillin solution and shook it, but no adduct crytals falled out.
Any idea what could be going wrong?
|
|
|
Sedit
International Hazard
    
Posts: 1939
Registered: 23-11-2008
Member Is Offline
Mood: Manic Expressive
|
|
Possibly your starting material is low in Vanillin content, what was the source of the Vanillin solution?
http://www.sciencemadness.org/talk/viewthread.php?tid=13974#...
Over the counter Vanillin products are rather useless for obtaining Vanillin. I have tried a few times for the hell of it and they all only contain a
few mg of Vanillin at best. Also you never added Alcohol to precipitate the Adduct and if you have read this thread you will see that the bisulfite
adducts are soluble in water.
Knowledge is useless to useless people...
"I see a lot of patterns in our behavior as a nation that parallel a lot of other historical processes. The fall of Rome, the fall of Germany — the
fall of the ruling country, the people who think they can do whatever they want without anybody else's consent. I've seen this story
before."~Maynard James Keenan
|
|
|
| Pages:
1
2 |