mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|
Gas-phase carbon deposits
Magnesium and dry ice can undergo a pyrotechnic redox reaction:
2Mg + CO2 -> 2 MgO + C
I assumed that this would be taking place in the gas phase, with burning magnesium vaporizing the metal and the dry ice, and the two reacting at high
temperature upon mixing. This made me wonder: what form would the solid carbon take?
So, I mixed ~ 50% stoichiometric excess powdered dry ice (a lot sublimes while igniting) with magnesium powder and lit it with a blow torch in an
aluminum weigh boat. I disgested the residue with hydrochloric acid for a couple days to remove magnesium oxide and any aluminum from the container,
then filtered and left to dry.
I photographed them with the SEM:

There's a lot of variation in the sorts of shapes, including crystals (sand contamination?) and various foamy, flakey grains:

I found one in particular to be interesting, with a sort of veined honeycomb structure:
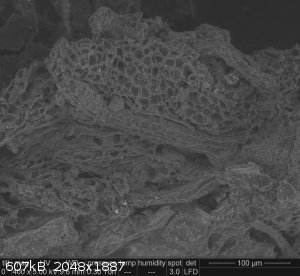 
Not sure if it tells me anything about the reaction processes... but it's cool looking!
al-khemie is not a terrorist organization
"Chemicals, chemicals... I need chemicals!" - George Hayduke
"Wubbalubba dub-dub!" - Rick Sanchez
|
|
|
chironex
Harmless

Posts: 40
Registered: 17-10-2016
Member Is Offline
Mood: No Mood
|
|
Huh that's actually some really cool looking structures. It looks a lot like the chemical vapour deposition reactions of carbon that tend to form
graphene and stuff. I'd bet a small quantity of that carbon is graphitic. It woulda been cool to see what sort of conductivity that material had. This
whole thing seems like a really interesting way to generate high surface area carbon structures.
|
|
|
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|
That is a good idea; I might make some more and find out. I wonder how I would standardize the measurement though? A line of loose powder vs. a
compacted pellet would likely have different values due to the difference in contact between particles, for example
al-khemie is not a terrorist organization
"Chemicals, chemicals... I need chemicals!" - George Hayduke
"Wubbalubba dub-dub!" - Rick Sanchez
|
|
|