| Pages:
1
2 |
420MLGnOhEADsCOPEpro
Harmless

Posts: 27
Registered: 22-1-2015
Member Is Offline
Mood: No Mood
|
|
sulfuric and hydrochloric acid generator design
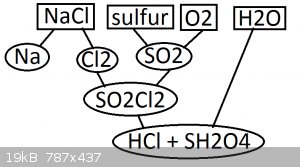
i plan to set up a system as such
electrolyse NaCl
http://en.wikipedia.org/wiki/Downs_cell
i have a kiln which can easily achieve the required temperature
the downs cell itself will be constructed with clay and the chlorine will be vented out of the kiln through borosilicate pipes
after the chlorine exits the kiln it may be cooled and transitioned to plastic pipe
sulfur dioxide is generated by simply burning sulfur
the sulfur dioxide is cooled in metal pipe then transitioned to plastic pipe
sulfur dioxide and chlorine gas are mixed 1:1 and forced through an activated carbon catylist where it reacts into sulfuryl chloride
http://en.wikipedia.org/wiki/Sulfuryl_chloride
sulfuryl chloride is bubbled through water where it reacts into hydrogen chloride and sulfuric acid
is there anything i've overlooked that may cause a problem or anything that might simplify the system or otherwise make it work better
|
|
|
Molecular Manipulations
Hazard to Others
  
Posts: 447
Registered: 17-12-2014
Location: The Garden of Eden
Member Is Offline
Mood: High on forbidden fruit
|
|
You remind me of myself, a couple years back.
It's really easy to do somthing like this, on paper, but when you actually start building it, you realize how much more difficult it is.
I get that you're trying to make/isolate all three chemicals (sodium, sulfuric and hydrochloric acid) but which do you want more? There's easier ways
to get all three of these chemicals than the methods proposed here, separately.
In theory it might work, but never without a strong, quality setup, pressure gauges, flow meter, regulators and a lot more thought and several hundred
dollars at least. It just won't be practical.
-The manipulator
We are all here on earth to help others; what on earth the others are here for I don't know. -W. H. Auden
|
|
|
420MLGnOhEADsCOPEpro
Harmless

Posts: 27
Registered: 22-1-2015
Member Is Offline
Mood: No Mood
|
|
that's exactly why i came here
if anybody has done something similar they probably at least have some idea of how to make the project go more smoothly
i'd like to buy the chemicals i need but i don't need enough to buy in bulk and otherwise they would cost hundreds or thousands of dollars
i have lots of free time and most of the materials i need so i figure it's reasonable to build something
|
|
|
j_sum1
Administrator
       
Posts: 6218
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
Keep it simple.
I would go for one product at a time. It is so easy to run into hurdles and you end up spending more time and money than you would if you simply
purchased the stuff. And you are likely to end up with low yield and/or low purity.
Multiply this by three for the triple system you are devising.
One thing to consider with your design is that if it does not work then you are missing tree products. On the other hand, if you have three separate
processes then one can fail and you are still producing the other two.
|
|
|
smaerd
International Hazard
    
Posts: 1262
Registered: 23-1-2010
Member Is Offline
Mood: hmm...
|
|
I think it's a cool idea. However, in order to do this you would essentially be setting up a miniature industrial manufacturing process to produce two
very common and widely available chemicals and sodium metal. Thinking about the costs of doing this "properly" makes my wallet shrivel considering I
go through a liter of either acid maybe every year. More power to you, have you started any more concrete plans for the design yet? Edit- I can
definitely see safety and fail-safes being important here. Those gasses are not friendly and are a serious concern on the mole+ scale.
The reactions look pretty standard so the chemistry is there, the 'engineering' is what comes next. Other-wise there isn't much to talk about.
[Edited on 24-1-2015 by smaerd]
|
|
|
420MLGnOhEADsCOPEpro
Harmless

Posts: 27
Registered: 22-1-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by j_sum1  | Keep it simple.
I would go for one product at a time. It is so easy to run into hurdles and you end up spending more time and money than you would if you simply
purchased the stuff. And you are likely to end up with low yield and/or low purity. |
i'll definitely test the systems one at a time before putting them together and i'm sure it will require quite some adjusting to get them running
together
(between making the sulfur burn at a somewhat steady rate and finding the right amperage for the electrolysis) but i've got lots of free time anyway
|
|
|
420MLGnOhEADsCOPEpro
Harmless

Posts: 27
Registered: 22-1-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by smaerd  | | I can definitely see safety and fail-safes being important here. Those gasses are not friendly and are a serious concern on the mole+ scale.
|
i'm thinking the following safety precautions are in order
>naturally safety goggles
>cloth wet with aqueous sodium bicarbonate to breath through (will react with gas before it gets to your lungs)
>wet ph tests all over the pipes (will change color if small concentrations of gas escape)
>a bucket of water (in the event the sulfur needs to stop burning)
>multiple places the electrolysis circuit can be broken (to prevent running wildly across the garage)
>testing all pipes for leaks (by means of bicycle pump)
|
|
|
Oscilllator
National Hazard
   
Posts: 659
Registered: 8-10-2012
Location: The aqueous layer
Member Is Offline
Mood: No Mood
|
|
I'm afraid one does not "simply burn sulfur." Taking a good long look through this thread will show that a sulfur burner is not an easy undertaking. The member axehandle tried many methods, each more crackpot than the last,
and I don't believe he ever succeeded.
|
|
|
420MLGnOhEADsCOPEpro
Harmless

Posts: 27
Registered: 22-1-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Oscilllator  | | I'm afraid one does not "simply burn sulfur." Taking a good long look through this thread will show that a sulfur burner is not an easy undertaking. The member axehandle tried many methods, each more crackpot than the last,
and I don't believe he ever succeeded. |
i had considered this might be difficult (though because i have no way to put enough air into a burning chamber not because sulfur burns poorly) and
have found a solution
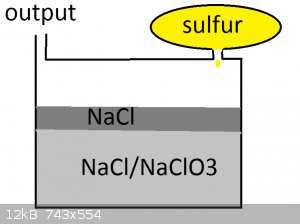
http://en.wikipedia.org/wiki/Sodium_chlorate
i can make oxidizer from sodium hydroxide and chlorine
thus allowing the design shown
>sulfur is slowly dripped into the salt
>sulfur reacts with NaCl / NaClO3 mix (the chlorate is diluted to prevent explosive burning and reduce gaseous sulfur (the top layer of NaCl is to
trap the rest of the gaseous sulfur))
>SO2 escapes through the output with some amount of pressure behind it (important for forcing it through the catylist)
|
|
|
Molecular Manipulations
Hazard to Others
  
Posts: 447
Registered: 17-12-2014
Location: The Garden of Eden
Member Is Offline
Mood: High on forbidden fruit
|
|
I've gotten a sulfur burner to work with only sulfur and oxygen.
I bubbled oxygen through liquid sulfur and lit the top with a torch.
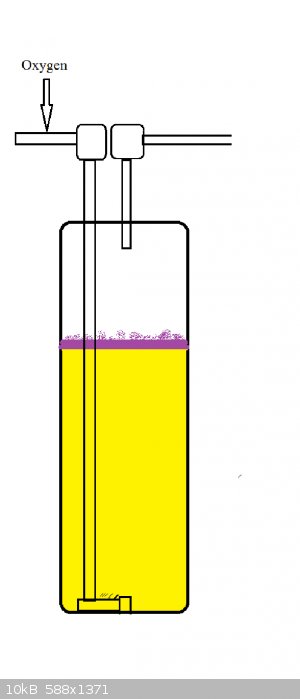
Not sure if it would work with air though. Why not try SO2 oxidation with O2 and V2O5 catalyst?
[Edited on 27-1-2015 by Molecular Manipulations]
-The manipulator
We are all here on earth to help others; what on earth the others are here for I don't know. -W. H. Auden
|
|
|
Loptr
International Hazard
    
Posts: 1347
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
I hope you have the elbow room for this undertaking. I can't imagine this would be very friendly for a residential area.
|
|
|
Fulmen
International Hazard
    
Posts: 1693
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
There is always something, isn't it? I've looked at most routes, but the only one that appeals to me is the contact process. Reading up it
seems like the V2O5-route is quite doable with the equipment I have available, and it all stops because you can't get sulfur to burn in air? Jeeez, is
somebody doing this on purpose?
|
|
|
Metacelsus
International Hazard
    
Posts: 2531
Registered: 26-12-2012
Location: Boston, MA
Member Is Offline
Mood: Double, double, toil and trouble
|
|
When I was working in a university lab, the grad students were doing some reactions using sulfuryl chloride (converting disulfides to sulfenyl
chlorides). Saying that stuff is nasty is putting it mildly. I'd call it face-melting.
|
|
|
420MLGnOhEADsCOPEpro
Harmless

Posts: 27
Registered: 22-1-2015
Member Is Offline
Mood: No Mood
|
|
i considered something of the sort (though with air instead of O2) but it think the flame might go out to easily because i don't intend to run this
thing particularly fast
@Loptr i have almost 5 acres or 20234.3 sq meters (142.25 meters square)
|
|
|
420MLGnOhEADsCOPEpro
Harmless

Posts: 27
Registered: 22-1-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Cheddite Cheese  | | When I was working in a university lab, the grad students were doing some reactions using sulfuryl chloride (converting disulfides to sulfenyl
chlorides). Saying that stuff is nasty is putting it mildly. I'd call it face-melting. |
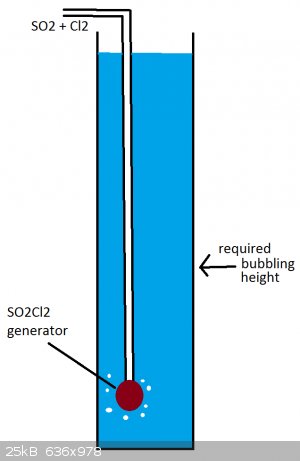
it's only a bit more than twice as bad as SO3 
i'll put the SO2Cl2 part in the bubbler (vvv like this) so it should be the safest part
|
|
|
Molecular Manipulations
Hazard to Others
  
Posts: 447
Registered: 17-12-2014
Location: The Garden of Eden
Member Is Offline
Mood: High on forbidden fruit
|
|
Melt the sulfur first, pour it in the burner, then turn on the air pump/oxygen cylinder and direct a torch flame to the sulfur. Always worked for me.
I then bubble the sulfur dioxide through water, through an ionizer to get rid of smoke particles and then through a calcium chloride tube to dry it.
I once used a few grams of vanadium pentoxide and tried out the Contact Process. It's not too hard but next time I'll buy the ready made catalysts.
-The manipulator
We are all here on earth to help others; what on earth the others are here for I don't know. -W. H. Auden
|
|
|
420MLGnOhEADsCOPEpro
Harmless

Posts: 27
Registered: 22-1-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Molecular Manipulations  | Melt the sulfur first, pour it in the burner, then turn on the air pump/oxygen cylinder and direct a torch flame to the sulfur. Always worked for me.
I then bubble the sulfur dioxide through water, through an ionizer to get rid of smoke particles and then through a calcium chloride tube to dry it.
I once used a few grams of vanadium pentoxide and tried out the Contact Process. It's not too hard but next time I'll buy the ready made catalysts.
|
if i were going to do the contact process i'd make a vanadium aero gel
http://www.aerogel.org/
|
|
|
Loptr
International Hazard
    
Posts: 1347
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
Quote: Originally posted by 420MLGnOhEADsCOPEpro  |
i considered something of the sort (though with air instead of O2) but it think the flame might go out to easily because i don't intend to run this
thing particularly fast
@Loptr i have almost 5 acres or 20234.3 sq meters (142.25 meters square) |
I am familiar with acres. 
I was just trying to make sure that this wasn't being done in an apartment or a crowded neighborhood. It seems you do have some privacy. I was just
thinking "what-if" in the event something went awry.
|
|
|
Fulmen
International Hazard
    
Posts: 1693
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
Where would you get smaller amounts of this? And how much difference in efficiency and life span would you expect? Or is it simply due to cost and
effort?
|
|
|
chief3
Harmless

Posts: 39
Registered: 29-12-2012
Member Is Offline
Mood: No Mood
|
|
Why dont you just try the following:
==> Step 1 : Get yourself some sulfate ... , eletrolyze it with a diaphragma ... : Redy there is some diluted H2SO4 ... ; this can be concentrated
by heat ... although some salt will remain in the concentrated resudual ; distillation of the concentrate might be possible to get quite clean H2SO4
...
==> Step 2: React the H2SO4 with NaCl ... , to have HCl - vapors ; those can be reacted with water to form HCl-acid .
|
|
|
Molecular Manipulations
Hazard to Others
  
Posts: 447
Registered: 17-12-2014
Location: The Garden of Eden
Member Is Offline
Mood: High on forbidden fruit
|
|
There's some at Alibaba. Most have a large minimum order, but a few are OK.
I don't think you can use pure vanadium pentoxide as a catalyst, it needs to be mixed with several other compounds, including cesium oxide and other
exotic chemicals. Buying it is much easier than making it.
-The manipulator
We are all here on earth to help others; what on earth the others are here for I don't know. -W. H. Auden
|
|
|
Fulmen
International Hazard
    
Posts: 1693
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
Nice, didn't even think of looking there. As for making catalysts, several people here has done it with apparently good results. Doesn't require more
than some ammonia and K2SO4 plus a substrate, but I agree that buying would be even simpler.
|
|
|
420MLGnOhEADsCOPEpro
Harmless

Posts: 27
Registered: 22-1-2015
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by chief3  | Why dont you just try the following:
==> Step 1 : Get yourself some sulfate ... , eletrolyze it with a diaphragma ... : Redy there is some diluted H2SO4 ... ; this can be concentrated
by heat ... although some salt will remain in the concentrated resudual ; distillation of the concentrate might be possible to get quite clean H2SO4
...
==> Step 2: React the H2SO4 with NaCl ... , to have HCl - vapors ; those can be reacted with water to form HCl-acid .
|
i have other projects that require chlorine gas and sodium metal is handy for some things too
also diaphragms
|
|
|
Molecular Manipulations
Hazard to Others
  
Posts: 447
Registered: 17-12-2014
Location: The Garden of Eden
Member Is Offline
Mood: High on forbidden fruit
|
|
Any progress yet 420pro? What material are you planning on using for pipes and tubes?
-The manipulator
We are all here on earth to help others; what on earth the others are here for I don't know. -W. H. Auden
|
|
|
420MLGnOhEADsCOPEpro
Harmless

Posts: 27
Registered: 22-1-2015
Member Is Offline
Mood: No Mood
|
|
after the gas is put through a heat exchange all the pipe will be pvc for convenience
before the heat exchange though i'm going to electroplate the inside of a piece of conduit in nickel which should then react into NiCl which is
chlorine resistant and solid up to 1000 C
if that doesn't work i'll use borosilicate pipe though borosilicate is more expensive, requires a larger heat exchanger, and is less easily coupled to
pvc (due to the difference in available diameter)
as for the sulfur burning part the high temperature pipes are aluminum and the rest are again pvc
recently i've been a bit side tracked with an aerogel project though both projects require chlorine gas so they should come together around he same
time
i'll be done with this and post results in 2-12 weeks
|
|
|
| Pages:
1
2 |