| Pages:
1
2 |
NitrousBoost
Harmless

Posts: 2
Registered: 30-1-2015
Member Is Offline
Mood: No Mood
|
|
1-(2-Thienyl)acetone?
Will it work, or does the acetone have to form an enolate? Maybe sodium hydroxide or sodium ethoxide?

|
|
|
Chemosynthesis
International Hazard
    
Posts: 1071
Registered: 26-9-2013
Member Is Offline
Mood: No Mood
|
|
First, excellent use of structural drawings. I find that representation extremely helpful in communicating a proposal, and it's easier for me to
consider questions in that type of format.
You want an enolate to initiate nucleophilic attack on an aromatic substrate? This is extremely unlikely to be favorable at any realistic conditions,
and there are only a handful of special cases where this happens under normal conditions. The thiophene has a large amount of conjugated pi system in
there that just makes it very hard to nucleophilicly assault.
What I would suggest as a reasonable alternative, though not hobby friendly, would be a Friedel Crafts acylation. Very standard, achievable
undergraduate intro organic chemistry reaction, and plenty of literature to follow.
An example can be found in J. Org. Chem., 1966, 31 (4), pp 1283–1285
DOI: 10.1021/jo01342a508
Your biggest methodological hurdle would be the obvious necessity of using halogenated acetone, which is lachrymatory to the point of being one of the
incipient chemical weapons of the early 20th Century.
|
|
|
turd
National Hazard
   
Posts: 800
Registered: 5-3-2006
Member Is Offline
Mood: No Mood
|
|
No. But at lest for iodobenzene it does work with cheap acetylacetone:
https://www.erowid.org/archive/rhodium/chemistry/p2p.acetyla...
I've also had some success with bromobenzene and the article posted by solo:
https://www.sciencemadness.org/whisper/viewthread.php?tid=16...
Thiophene is more electron rich so you might have to do some optimization.
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Chemosynthesis  | | First, excellent use of structural drawings. I find that representation extremely helpful in communicating a proposal, and it's easier for me to
consider questions in that type of format. |
how do you draw such structures ? what software do you use.please tell me
| Quote: | | You want an enolate to initiate nucleophilic attack on an aromatic substrate? This is extremely unlikely to be favorable at any realistic conditions,
and there are only a handful of special cases where this happens under normal conditions. The thiophene has a large amount of conjugated pi system in
there that just makes it very hard to nucleophilicly assault |
maybe we could make use of the branch of chemistry designed for such "impossible" reactions-umpolung
http://en.wikipedia.org/wiki/Umpolung
I am not smart enough to tell you how to use it though
maybe lord nicodem could give us some advice 
| Quote: | | What I would suggest as a reasonable alternative, though not hobby friendly, would be a Friedel Crafts acylation |
chemo,don't you mean alkylation ? and why do you say F.C is not hobby friendly.do you mean the use of AlCl3 or FeCl3?
since thiopene is more activated than benzene,I think weaker lewis acids like SnCl4 or ZnCl2 could be used
| Quote: | | Your biggest methodological hurdle would be the obvious necessity of using halogenated acetone, which is lachrymatory to the point of being one of the
incipient chemical weapons of the early 20th Century |
I recently found out it was carcinogenic as well
http://www.sciencemadness.org/talk/viewthread.php?tid=61289&...
the acetoacetate idea looks good,but I noticed that you would need a stream of N2 gas,why ?
maybe you could do it in a method similar to the allylbenzene-->>>P2P using wacker oxidation
UTSE for ways to make allylbenzene
| Quote: | | Will it work, or does the acetone have to form an enolate? Maybe sodium hydroxide or sodium ethoxide? |
maybe,if you used soda amide or n-butyllithium
another reaction,just to explore all possibilities
http://en.wikipedia.org/wiki/Meerwein_arylation
see under scope
[Edited on 31-1-2015 by CuReUS]
|
|
|
Chemosynthesis
International Hazard
    
Posts: 1071
Registered: 26-9-2013
Member Is Offline
Mood: No Mood
|
|
Yes, I meant to say alkylation. Thank you. I say it is not friendly to a hobbyist (in this instance) due to the use of haloacetone, which is acutely
fairly toxic as well, even though sometimes F.C. can be fairly Lewis acid specific, which may be superficially problematic to those unwilling or
unable to replicate Chainhit's "thug furnace." Can you cite an example with tin or a similar substitute? Haloacetones are less reactive than typical
F.C. reagents.
As an aside, a F.C. is an umpolung relative the enolate.
Using a strong, non-nucleophilic base wouldn't overcome the harshness of trying to add electrons to a nucleophilic, aromatic ring.
If the poster proposed this reaction for us, my opinion is for them to study the classic introductory reactions first before moving on to other
wonderful suggestions.
[Edited on 31-1-2015 by Chemosynthesis]
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
yesterday night I had a breakthrough,I have found the perfect reaction for you
this reaction works for bromobenzene,so I think it should work here too.It is basically a beta-keto ester decarboxylation.The reaction goes like this:
make sodium ethoxide first,and to that add a mixture of ethylacetoacetate(EAA) and bromobenzene
you will get a beta-keto ester.Treat this with H2SO4 .The acid decomposes the beta keto ester to give CO2 and your
aryl ketone,which in this case is phenylacetone
| Quote: | PHENYLACETONE FROM B-KETO ESTERS
--------------------------------------------------------------------------
In this chapter, I will cover two separate but similar methods of
making phenylacetone. Neither of them is actually suitable for
industrial-scale production, but they have the advantage of not using
phenylacetic acid. This allows an underground chemist to diversify the
chemicals used, and enables him to defeat a blockade on his phenylacetic
acid supply. Neither of these reactions is foolproof; both require a
certain amount of laboratory skill. The chemicals must be weighed and
measured fairly exactly. This is unlike the method described in Chapter 3,
where anything within a ballpark range will work. These methods require a
reliable scale.
Both of these reactions use sodium metal, which is some nasty stuff. It
reacts violently with water to produce sodium hydroxide and hydrogen. It
will also react with air. The chemist never touches it intentionally; if he
does touch it, he washes it off with warm water. Sodium metal comes in a
can, covered with a bath of petroleum distillate. This is to protect it
from water and air. As long as it stays covered, it causes the chemist no
problems.
In this reaction, sodium metal is reacted with absolute alcohol to make
sodium ethoxide (NaOCH2CH3). Ethyl acetoacetate and bromobenzene are then
added to this to produce a beta keto ester. Reaction with acid then
produces phenylacetone.
A side reaction which sometimes becomes a problem is bromobenzene
reacting with beta keto ester to produce di-phenylacetone. This can be
controlled by not using too much bromobenzene, adding it slowly and
stirring it well.
Figure 12 shows the glassware used. The glassware must be very dry, so
it is dried out in the oven for an hour or so. If the sep funnel has a
plastic valve, the valve is taken out before the sep funnel is put in the
oven. The magnetic stirring bar does not go in the oven either. It is
coated with Teflon, so it does not have any water on it. A magnetic stirrer
is necessary to do this reaction, because good stirring is very important.
An extra claisen adapter is needed for this reaction; one is filled with
broken pieces of glass for use as a fractionating column, the other is kept
as is for use in the Figure 12 apparatus.
To begin, the underground chemist puts a bed of Drierite in the vacuum
adapter as shown in Figure 2a, being sure to plug up the vacuum nipple. The
water lines are attached to the condenser and cold water started flowing
through it. But if it is humid, the water flow is not started until the
glassware is assembled.
The can of sodium is opened. A chunk about the size of a medium egg is
needed. The chemist selects a convenient corner of the block of sodium to
work on. With a clean, sharp knife, he scrapes off any discolored skin
there might be in the area he plans to use. Good clean sodium has a bright
metallic look. He keeps the block under the petroleum as he scrapes the
discolored skin.
Now he must weigh the sodium. A 100 ml beaker is filled halffull of the
petroleum distillate from the can of sodium, or with xylene. He puts it on
the scale and weighs it. He needs 34.5 grams of sodium metal, so with a
clean sharp knife. he cuts off a chunk of sodium, transfers it to the
beaker and weighs it. If it is not quite 34.5 grams, he cuts a little more
sodium and adds it to the beaker. This is done quickly, so that evaporation
of the petroleum does not throw the measurement off. Then another 100 ml
beaker is filled half-full of anhydrous ethyl ether. The sodium metal is
transferred to it with a spoon. The petroleum is poured back in with the
block of sodium and the can sealed up so that it does not evaporate. With a
clean sharp knife, the sodium is cut up into little pieces about 1/2 the
size of a pea.
The sodium is kept under the ether while this is being done. Eye
protection is always worn when working with sodium.
After the sodium is cut up, the magnetic stirring bar is put in the
2000 ml flask. Then the sodium metal pieces are scooped out with a spoon
and put in the 2000 ml flask. The glassware is immediately assembled as
shown in Figure 12. One liter (1000 ml) of absolute ethyl alcohol is
measured out. Absolute alcohol absorbs water out of air, so this is done
rapidly. Here's how. The chemist gets a quart beer bottle, marks on the
outside how full one liter is, and bakes the bottle in the oven to dry it
out. When he takes it out of the oven, he sucks the hot, moist air out of
it with a section of glass tubing. Once it has cooled down, he fills it
with one liter of absolute alcohol and stoppers it to keep it dry. He wants
to get the alcohol in with the sodium before the ether on it evaporates,
and this saves him the time of measuring it out.
About 200 ml of the absolute alcohol is put in the sep funnel and the
valve opened to allow the alcohol to flow down onto the sodium metal. Cold
water should be flowing through the condenser. Magnetic stirring is not
necessary at this time, but the 2000 ml flask is sitting in a large pan. A
pail of cold water and a towel are kept handy. Sodium and alcohol react
together vigorously, and the alcohol boils like crazy. The condenser is
checked to see how far up the alcohol vapors are reaching. The chemist does
not want the alcohol vapors to escape out the top of the condenser. If the
vapors are making it more than halfway up the condenser, cold water is
poured from the pail into the pan the flask is sitting in. That cools it
off and slows down the boiling. But if that does not do enough, the wet
towel is put on top of the flask. When the boiling slows down, the towel
and the pan of water are removed, then more alcohol is added to the sep
funnel. A fresh ball of cotton is put in the top of the sep funnel to
protect the alcohol from water in the air. The alcohol is added to the
flask at such a Mte that the boiling of the alcohol continues at a nice
Mte. When all of the original one liter of absolute alcohol has been added
to the flask, the flask is gently heated on the hot plate to keep the
alcohol boiling until the little pieces of sodium are dissolved. If the
chemist has done a very good job, the result is a clear solution. If not,
it will be milkycolored.
The magnetic stirring is now begun, and 195 grams (190 ml) of
ethylacetoacetate is put in the sep funnel over the next 15 minutes. The
solution is heated to a gentle boiling. As it is boiling and stirring, 236
grams of bromobenzene is put in the sep funnel and dripped into it over a
period of an hour. The boiling and stirring is continued for 8 hours.
Then the stirring is stopped and the solution allowed to cool down. A
good amount of sodium bromide crystals settle to the bottom of the flask.
When they have settled to the bottom, the glassware is taken apart and as
much of the alcohol solution as possible is poured into a 3000 ml flask.
The last of the product is rinsed off the sodium bromide crystals by adding
about 50 ml of absolute alcohol to them, swirling around the mixture, then
filtering it. This alcohol is added to the alcohol in the 3000 ml flask.
The glassware is set up as shown in Figure 3 in Chapter 3. A 1000 ml
flask is used as the collecting flask. The alcohol in the 3000 ml flask is
heated. The oil in the pan is not heated above 115ø C. The distillation is
continued until the chemist has collected over 900 ml of alcohol in the
collecting flask.
When the alcohol has been boiled out, the heat is turned off and the
flask removed from the pan of oil. As it is cooling off, 1500 ml of 5%
sodium hydroxide solution is mixed. To do this, 75 grams of sodium
hydroxide is put in a flask and 1400 ml of water added. (Lye may be used as
a sodium hydroxide substitute.) When both the sodium hydroxide solution and
the reaction mixture near room temperature, the sodium hydroxide solution
is poured into the 3000 ml flask with the reaction mixture. The magnetic
stirring bar is put into the flask and magnetic stirring is begun. It is
stirred fast enough that a whirlpool develops in the mixture and the~beta
keto ester gets into contact with the sodium hydroxide solution. The
stirring is continued for 4 hours without heating the solution. The beta
keto ester reacts with the sodium hydroxide to produce the compound shown
above, plus ethyl alcohol. This is a hydrolysis reaction.
After 4 hours of stirring, the stirring is stopped and the solution
allowed to sit for a few minutes. A small amount of unreacted material will
float up to the top. If there is a large amount of unreacted material, the
stirring is begun again and 40 grams of sodium hydroxide and 300 ml of
isopropyl rubbing alcohol are added. It is stirred for 4 more hours. But
generally this is not necessary.
The unreacted layer is poured into a 1000 ml sep funnel. A good deal of
the sodium hydroxide solution will be poured off with it. The chemist lets
it sit for a few minutes, then drains the sodium hydroxide solution back
into the 3000 ml flask. The oily unreacted material is poured into a small
glass bottle and kept in the freezer. When a good amount of it has
accumulated, the chemist tries reacting it again with 5% sodium hydroxide
solution. However, this will not yield very much more product, because most
of this oily material is the diphenylacetone byproduct.
The underground chemist is now ready to produce phenylacetone. The
compound shown above will react with sulffiuric acid to produce
phenylacetone and carbon dioxide gas. He mixes up 150 ml of 50% sulffiuric
acid. To do this, he adds slightly more than 55 ml of sulfuric acid to
slightly less than 105 ml of water; if he added more sodium hydroxide and
alcohol to his reaction mixture, he mixes up twice as much 50% sulfuric
acid.
The stirrer in the 3000 ml flask containing the sodium hydroxide is
started up again. Then the 50% sulffiuric acid is slowly added to it. It
will bubble out carbon dioxide like crazy and crystals of sodium sulfate
will be formed. Phenylacetone will also be formed, some of it floating on
the surface of the solution, some of it trapped among the crystals formed.
When all of the sulffiuric acid has been added, and the bubbling of carbon
dioxide has slowed down to just about stopping, the stirring is stopped.
The glassware is set up as shown in Figure 3. The collecting flask is
2000 ml. The 3000 ml flask is slowly heated to boiling. The steam carries
the phenylacetone along with it to the other flask. This process is called
a steam distillation. The distilling is continued until a little more than
1000 ml is in the collecting flask. By then, almost all the phenylacetone
will be carried over into the collecting flask. There will be two layers in
the collecting flask, a yellow layer of phenylacetone on top, and a clear
water layer. There will be some acid dissolved in the water. Forty grams of
sodium hydroxide is dissolved in 150 ml of water, then added to the 2000 ml
flask. The flask is stoppered and shaken for one minute to destroy the
acid. Then 100 ml of benzene is added to the flask and it is shaken some
more. The phenylacetonebenzene layer is poured into a 1000 ml sep ffiunnel
and allowed to sit for a couple of minutes. Then the water layer is drained
off back into the 2000 ml flask. The phenylacetone layer is poured into a
500 ml flask along with a few boiling chips. Then 100 ml of benzene is
added to the 2000 ml flask, which is shaken again for about 30 seconds
before it is allowed to sit for a few minutes. The benzene layer is poured
into the 1000 ml sep funnel and allowed to sit for a couple of minutes. The
water layer is drained out, and the benzene layer is poured into the 500 ml
flask with the rest of the phenylacetone. The glassware is set up as shown
in Figure 5 and the phenylacetone distilled as described in Chapter 3. The
yield is about 125 ml of phenylacetone. (For more information on thisreaction, see Organic Reactions, Volume 1, published in 1942, page 266.)
Secrets of Methamphetamine Manufacture (3rd ed.)
by Uncle Fester |
EAA can be easily made http://en.wikipedia.org/wiki/Ethyl_acetoacetate#Preparation
also to make allylthiopene,treat 2-iodothiopene with Mg to make a grignard reagent and then react it with allylbromide to get allylthiopene
then do wacker oxidation to get your ketone
http://en.wikipedia.org/wiki/Wacker_process#Wacker.E2.80.93T...
| Quote: | | sometimes F.C. can be fairly Lewis acid specific, which may be superficially problematic to those unwilling or unable to replicate Chainhit's "thug
furnace." |
I didn't understand what you said,please tell again
| Quote: | | Can you cite an example with tin or a similar substitute? |
http://www.uio.no/studier/emner/matnat/kjemi/KJM5220/h07/und...
read Pg 4
But nitrous boost,please do the reaction,this shouldn't be an example of mental masterbation or fantasizing
|
|
|
Nicodem
Super Moderator
      
Posts: 4230
Registered: 28-12-2004
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by CuReUS  | But nitrous boost,please do the reaction,this shouldn't be an example of mental masterbation or fantasizing |
Let me guess, is it because you don't want this topic to remain a mental masturbation that you decided to spam it with Uncle Fester's mental
masturbations?
…there is a human touch of the cultist “believer” in every theorist that he must struggle against as being
unworthy of the scientist. Some of the greatest men of science have publicly repudiated a theory which earlier they hotly defended. In this lies their
scientific temper, not in the scientific defense of the theory. - Weston La Barre (Ghost Dance, 1972)
Read the The ScienceMadness Guidelines!
|
|
|
Chemosynthesis
International Hazard
    
Posts: 1071
Registered: 26-9-2013
Member Is Offline
Mood: No Mood
|
|
Turd's post of the acetoacetone and article solo had was better from the standpoint of avoiding yield loss with a subsequent Wacker oxidation, and the
expense of catalyst. Similar concept, though.
There was a sciencemadness thread posted way back about making aluminum trichloride with a crude furnace. One of the members I often wonder about,
since we used to post on the same forum on another website.
Good link, however, the problem is that your link notes that alkylation is "not good react" here, in contrast with the acylation. Given haloalkanes'
relative reactivity to many other alkylations, I would be surprised if this were viable with tin. It might not be with ferrous or aluminum chlorides
either, but they would be more likely if that were the scheme tested.
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Nicodem  |
Let me guess, is it because you don't want this topic to remain a mental masturbation that you decided to spam it with Uncle Fester's mental
masturbations? |
you had humiliated uncle fester in another thread too.I checked out the acetoacetic ester reaction before posting and its right
http://matematicas.udea.edu.co/~carlopez/Organic_Name_Reacti...
even eleusis couldn't tame fester
https://www.erowid.org/archive/rhodium/chemistry/eleusis/ele...
this is what eleusis had to say about the beta-keto ester method in fester's book
| Quote: | Chapter 7
This is actually a pretty clever way of making P-2-P, but let's face it - a sodium alkoxide reduction is *not* within the grasp of the average
chemical tinkerer. This is potentially dangerous stuff, you have been warned. Also, a ball of cotton is not an acceptable method of excluding water
vapor from getting into the reaction unless you live in a desert region. Use an appropriate drying tube packed with Calcium Chloride or Sodium
Hydroxide. |
If you say eleusis is a fool too,you will surely get stung
[Edited on 3-2-2015 by CuReUS]
|
|
|
Loptr
International Hazard
    
Posts: 1347
Registered: 20-5-2014
Location: USA
Member Is Offline
Mood: Grateful
|
|
Where did he end up? Prison?
That would be my definition of a fool.
|
|
|
Chemosynthesis
International Hazard
    
Posts: 1071
Registered: 26-9-2013
Member Is Offline
Mood: No Mood
|
|
Jenkins admitted to ordering all kinds of chemicals and glassware, including precursors, in his real name, with his personal credit card, to his
places of residence... knowing this was probably insanely stupid. I'd say that's foolish, regardless of how much determination and intellect it took
to teach himself a decent amount of organic chemistry as an English major.
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
that's not the point.He didn't want to run a drug empire,he only wanted the experience
and that was not the reason why he got caught.It was because of his stupid GF
https://www.erowid.org/archive/rhodium/chemistry/eleusis/mem...
anyways,my intention of mentioning eleusis was to show nicodem that he is not the only one who knows chemistry and he has no right to humiliate people
before doing a background search
I am sure nicodem has not even read uncle fester's secret of meth manufacture.He must have stumbled across a thread in the hive by an incompetant and
idiotic wannabe meth cook who had tried to replicate fester's instructions and failed and was complaining about it
If nicodem is so smart,why doesn't he provide a synthesis route to 1-(2-Thienyl)acetone?
[Edited on 3-2-2015 by CuReUS]
|
|
|
Chemosynthesis
International Hazard
    
Posts: 1071
Registered: 26-9-2013
Member Is Offline
Mood: No Mood
|
|
I don't see how purported motivation cancels out admitted flagrant violations of the law with no regard for the ramifications.
It was still foolish, and to say he was
caught because of his girlfriend when law enforcement had intercepted his package earlier is questionable.
Preisler does have a chemistry background with his undergraduate degree and experience in methamphetamine manufacture, but he is not above criticism,
and there remains a lot of criticism of his works, on Amazon, wikipedia, and elsewhere. Whether it's relevant to the portion you posted, or if the
reaction is particularly viable for thiophenes, I can't say.
|
|
|
Darkstar
Hazard to Others
  
Posts: 279
Registered: 23-11-2014
Member Is Offline
Mood: Sleepy
|
|
If you're up to it, what about a Grignard reaction between (thiophen-2-yl)magnesium iodide and propylene oxide? This would give a secondary alcohol
(after an acidic workup) that could be oxidized to your ketone. Or, instead of oxidizing it and doing reductive amination, you could convert the
alcohol to an alkyl bromide/iodide and try aminating that instead.
Forgive me if I'm mistaken, but I'm assuming your target molecule is thiopropamine/methiopropamine? You don't have to answer. Just something to
consider if you're seriously planning on trying this synthesis.
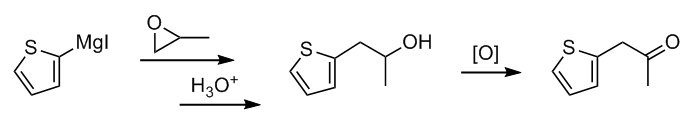
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Darkstar  | | If you're up to it, what about a Grignard reaction between (thiophen-2-yl)magnesium iodide and propylene oxide? This would give a secondary alcohol
(after an acidic workup) that could be oxidized to your ketone |
I might be wrong but if you try to oxidise the secondary alcohol,the sulphur too might get oxidised to form sulphones and sulphoxides
a mild oxidising agent like bleach cannot be used as the ketone is a methyl ketone so haloform reaction will take place and you will end up with
chloroform
| Quote: | | Forgive me if I'm mistaken, but I'm assuming your target molecule is thiopropamine/methiopropamine? You don't have to answer |
I dont think he is going to try the synthesis at all.Mostly this is a homework problem ,that's why he posted it in "beginning" and not in the organic
chemistry section
BTW,how did you draw the strcutures
[Edited on 3-2-2015 by CuReUS]
|
|
|
Darkstar
Hazard to Others
  
Posts: 279
Registered: 23-11-2014
Member Is Offline
Mood: Sleepy
|
|
Quote: Originally posted by CuReUS  |
I might be wrong but if you try to oxidise the secondary alcohol,the sulphur too might get oxidised to form sulphones and sulphoxides
|
Thiophenes are actually quite resistant to oxidation. Remember that it's an aromatic system.
| Quote: |
a mild oxidising agent like bleach cannot be used as the ketone is a methyl ketone so haloform reaction will take place and you will end up with
chloroform |
There are plenty of other options, though. (some available to the home chemist, some not) One that's mild and selective towards secondary alcohols
would be preferable.
| Quote: |
I dont think he is going to try the synthesis at all.Mostly this is a homework problem ,that's why he posted it in "beginning" and not in the organic
chemistry section |
Seems like a strange homework problem, though, given that it's an obvious drug precursor and all. Who knows... Maybe he has a cool professor? Either
way, Grignards aren't exactly all that easy to pull off anyway.
| Quote: |
BTW,how did you draw the strcutures |
ChemDraw Pro.
|
|
|
Chemosynthesis
International Hazard
    
Posts: 1071
Registered: 26-9-2013
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by CuReUS  |
I dont think he is going to try the synthesis at all.Mostly this is a homework problem ,that's why he posted it in "beginning" and not in the organic
chemistry section
|
I'm of the opinion he should post in Beginnings regardless because he has no references, and is clearly not yet capable of further synthesis towards a
grey market/research chemical target product.
|
|
|
AvBaeyer
National Hazard
   
Posts: 644
Registered: 25-2-2014
Location: CA
Member Is Offline
Mood: No Mood
|
|
"a mild oxidising agent like bleach cannot be used as the ketone is a methyl ketone so haloform reaction will take place and you will end up with
chloroform"
Secondary alcohols oxidize to ketones in high yields with sodium hypochlorite under acidic conditions. See RV Stevens, et al., J. Org. Chem., Vol. 45,
No. 10, 1980, 2030 and references cited therein. Thus, the Gignard reaction with propylene oxide followed by oxidation (acidic hypochlorite or
alternatives) would provide the desired ketone. Thiophene will be stable to most oxidants.
AvB
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
actually,I am not well versed with sulphur chemistry
do oxidising agents like KMnO4,chromtes etc oxidise S to sulphoxides and sulphones ?
The only oxidising agent that I know of which does that is H2O2
| Quote: | | There are plenty of other options, though. (some available to the home chemist, some not) One that's mild and selective towards secondary alcohols
would be preferable |
could you tell some ? The only ones that came to my mind was the oppenauer oxidation,lead tetracetate(but this might methylate thiopene),lead
nitrate(not sure about this one,but I read that it was an alternative to hexamine for the sommlet reaction),ferric ferrocyanide(from printer ink,I am
not sure about this one too ) ) Quote: Originally posted by AvBaeyer  |
Secondary alcohols oxidize to ketones in high yields with sodium hypochlorite under acidic conditions. See RV Stevens, et al., J. Org. Chem., Vol. 45,
No. 10, 1980, 2030 and references cited therein. |
and you please see this
http://en.wikipedia.org/wiki/Haloform_reaction
| Quote: | | Thus, the Gignard reaction with propylene oxide followed by oxidation (acidic hypochlorite or alternatives) would provide the desired ketone.
Thiophene will be stable to most oxidants. |
acidic ? I thought hypochorites were always used in alkaline medium
[Edited on 4-2-2015 by CuReUS]
|
|
|
Darkstar
Hazard to Others
  
Posts: 279
Registered: 23-11-2014
Member Is Offline
Mood: Sleepy
|
|
The haloform reaction only occurs under basic conditions. AvBaeyer is correct about secondary alcohol-to-ketone oxidations using sodium
hypochlorite/hypochlorous acid. Such oxidations are usually carried out in GAA.
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
in the acetaldehyde thread,one member had given the idea of using bleach on ethanol to make acetaldehyde but another member had warned him about the
haloform reaction and also said that hypochlorites could only be used in alkaline medium
then bleach is a good choice for the oxidation
but what are the other potential oxidising agents ?
[Edited on 4-2-2015 by CuReUS]
|
|
|
DJF90
International Hazard
    
Posts: 2266
Registered: 15-12-2007
Location: At the bench
Member Is Offline
Mood: No Mood
|
|
Do your own book work Cureus, a good undergraduate text will provide you with a plethora of options.
|
|
|
Darkstar
Hazard to Others
  
Posts: 279
Registered: 23-11-2014
Member Is Offline
Mood: Sleepy
|
|
Here are a few off the top of my head to get you started:
Chromium trioxide (Jones oxidation)
Dichromates
Permanganates
Manganese dioxide
PCC
DMP
Oxalyl chloride/DMSO (Swern oxidation)
Aluminium isopropoxide/acetone (Oppenauer oxidation)
H2O2 (with phase-transfer catalyst)
This list is by no means exhaustive. There are many, many more potential oxidizing agents/methods out there. 
|
|
|
AvBaeyer
National Hazard
   
Posts: 644
Registered: 25-2-2014
Location: CA
Member Is Offline
Mood: No Mood
|
|
CuReUs
Here is the Stevens paper. Read it and absorb!
AvB
Attachment: ketones via hyypochlorite_stevens_JOC_1980[1].pdf (461kB)
This file has been downloaded 448 times
|
|
|
CuReUS
National Hazard
   
Posts: 928
Registered: 9-9-2014
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Darkstar  | Here are a few off the top of my head to get you started:
Chromium trioxide (Jones oxidation)
Dichromates
Permanganates
Manganese dioxide
PCC
DMP
Oxalyl chloride/DMSO (Swern oxidation)
Aluminium isopropoxide/acetone (Oppenauer oxidation)
H2O2 (with phase-transfer catalyst)
This list is by no means exhaustive. There are many, many more potential oxidizing agents/methods out there.  |
thank you,but these are the standard oxidising agents.No one nowadays wants to work with chromium because its too toxic,permaganate had been put on
the schedule 2 list,MnO2 is generally suitable for allylic and benzylic hydroxyl groups and thiopenyl propan-2-ol is neither.even then the
yield is not good(see the benzaldehyde thread)
PCC,PDC,DMP etc are the home chemist's elusive dreams and the H2O2/HCOOH method creates a mess
if you ask me,the only one I see that can be used by a home chemist is the swern oxidation,but even then working with oxalyl chloride might be
dangerous,and if you try to make oxalyl chloride at home,you must keep in mind that one of the side products is phosgene
that's why I suggested using bleach because its cheap,gived high yield and is easily available
I was looking for out-of-the-box oxidising methods,that some members here know of.
DFJ90 wrongly assumed that I wanted to be spoonfed
| Quote: | | Here is the Stevens paper. Read it and absorb! |
beautiful. I think chemistry will never fail to surprise me I think chemistry will never fail to surprise me
[Edited on 5-2-2015 by CuReUS]
|
|
|
| Pages:
1
2 |