xfusion44
Hazard to Others
  
Posts: 223
Registered: 6-8-2014
Location: Europe
Member Is Offline
Mood: Nostalgic
|
|
Production of sodium oxide
Hi,
I would like to make sodium oxide, from sodium carbonate, by heating it. Does anyone know, how much time is required for Na2CO3 to decompose, when its
heated a little bit above its melting point? I've already managed to melt it, but I don't know how much time it needs to be molten, to fully
decompose. I melted it, using a clay oven and I left it inside for a few minutes in molten state, just to see, if anything happens, but after weighing
it, there was no change in weight (as I've expected). So, I've tried leaving it in there for 8 hours and I came back to see a puddle of hard Na2CO3
(Na2O?) sticked to the bottom of oven and a very weakened cup, which used to be hard inox cup. Cup was brittle almost like graphite (and it also
looked like it) - I've had a same problems one time, when using tin can for melting zinc. Also my oven isn't working anymore (I don't know, if this
has something to do with molten compound or it was just because of heat). Oven was very old - maybe it's just the heater wire that has failed... I'd
try this again with butane torch, but I need some data about the time needed for this decomposition to happen.
Thanks!
[Edited on 28-2-2015 by xfusion44]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Thermal decomposition of Group 1 carbonates requires very high temperatures, well above Bunsen for instance, and prolonged times. And then there's the
problem of keeping Na2O as Na2O...
These oxides aren't really to be compared with 'ordinary' oxides.
|
|
|
xfusion44
Hazard to Others
  
Posts: 223
Registered: 6-8-2014
Location: Europe
Member Is Offline
Mood: Nostalgic
|
|
Hmm, this seems like no go 
Well, if anyone has some other ideas for making Na2O, please post them 
blogfast25, how do you mean that Na2O is hard to store? Is it reactive with moisture in the air?
Thanks!
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Moisture and CO2 of course.
|
|
|
Molecular Manipulations
Hazard to Others
  
Posts: 447
Registered: 17-12-2014
Location: The Garden of Eden
Member Is Offline
Mood: High on forbidden fruit
|
|
I once decomposed potassium nitrate. It was not easy, a oxyacetylene torch was used, first I melted it and continued heating well past red-hot, my
guess is it was over 1,300 deg. C. After about 15 minutes I measured a weight loss of about 5% only. Now since potassium peroxide is what likely
formed that accounts for a little more decomposed nitrate. At first I assumed it only decomposed to nitrite but it was basic in solution and gave off
oxygen IIRC, which is why I think it was potassium peroxide. Still it was not at all of any use and I haven't tried it since.
-The manipulator
We are all here on earth to help others; what on earth the others are here for I don't know. -W. H. Auden
|
|
|
xfusion44
Hazard to Others
  
Posts: 223
Registered: 6-8-2014
Location: Europe
Member Is Offline
Mood: Nostalgic
|
|
Yeah, some compounds are extremely hard to decompose by heating. Last thing before Na2CO3, that I was trying to decompose, was CuSO4. I wanted to make
sulfuric acid by heating it pass 560°C and bubbling resulting SO3 gas through water, but that didn't work very well - it did decompose, but the SO3
gas was just too nasty and I decided to stop...
|
|
|
Zyklon-A
International Hazard
    
Posts: 1547
Registered: 26-11-2013
Member Is Offline
Mood: Fluorine radical
|
|
Yeah, you don't bubble sulfur trioxide into water. That will land you with a hot mess of broken glassware, dangerous fumes and dilute sulfuric acid
spilled everywhere. You need a ground glass setup, and you must lead the sulfur trioxide into concentrated sulfuric acid, not water!
|
|
|
xfusion44
Hazard to Others
  
Posts: 223
Registered: 6-8-2014
Location: Europe
Member Is Offline
Mood: Nostalgic
|
|
Quote: Originally posted by Zyklon-A  | | Yeah, you don't bubble sulfur trioxide into water. That will land you with a hot mess of broken glassware, dangerous fumes and dilute sulfuric acid
spilled everywhere. You need a ground glass setup, and you must lead the sulfur trioxide into concentrated sulfuric acid, not water!
|
That fortunately couldn't happen, because this was a test tube scale experiment, outside and with all necessary protection. I think that one would
need a lot bigger quantities of SO3 for such thing to happen. SO3 gas was produced really slowly, when CuSO4 was heated, so this experiment was far
from explosive one. Even if you for example add water to 98% H2SO4, there will be no dangerous reaction, untill you add too much of water at once, I'd
say, that the same holds true for SO3/water example.
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|

|
|
|
Zyklon-A
International Hazard
    
Posts: 1547
Registered: 26-11-2013
Member Is Offline
Mood: Fluorine radical
|
|
It doesn't matter the scale so much as the apparatus. You can't use any plastic or rubber tubing, it will instantly burn through any non-fluoranated
rubber. Ground glass or Teflon is a must.
If you think adding water to sulfuric acid is in any way comparable to sulfur trioxide you are in for a shock. A spec of solid sulfur trioxide added
to a test tube of water will likely explode it.
|
|
|
woelen
Super Administrator
        
Posts: 7977
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I can confirm the extremely violent reaction between SO3 and water. I once poured a few ml of 20% oleum in cold water and all I can say is WOW! The
reaction is ultra violent, produces a lot of crackling noise and unbelievable amounts of heat. In comparison, the reaction between sodium and water is
tame and even the reaction between potassium and water is less scary than the one between SO3 and water.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Interestingly even Sigma Aldrich only markets one grade of Na2O - 80 % (the rest being mainly Na2O2):
http://www.sigmaaldrich.com/catalog/product/aldrich/645613?l...
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
I know the decomposition of sodium nitrate has been mentioned, but what about sodium nitrite?
According to wiki on sodium nitrite, this occurs at 330°C, so 'red heat' should be ample.
2 NaNO2 → Na2O + NO + NO2 (1)
Unfortunately, the gases generated are toxic, but if they can be collected and bubbled through a sodium hydroxide solution, you could recycle them and
effectively be making sodium oxide from hydroxide by employing:
2 NaOH + NO2 + NO → 2 NaNO2 + H2O (2)
References:
(1) Stern, Kurt H. (1972). "High Temperature Properties and Decomposition of Inorganic Salts; Part 3. Nitrates and Nitrites". J. Phys. Chem. (US Naval
Research Laboratory) 1 (3): 750–751.
(2) http://en.wikipedia.org/wiki/Sodium_nitrite
[Edited on 1-3-2015 by deltaH]
|
|
|
Molecular Manipulations
Hazard to Others
  
Posts: 447
Registered: 17-12-2014
Location: The Garden of Eden
Member Is Offline
Mood: High on forbidden fruit
|
|
I can almost guarantee you from experience that 330 deg. C is simply bullshit. I've tried many times and never had any success at even 800 deg. C
decomposing sodium nitrite. A solution of the product of heating sodium nitrite had about the same pH as pure sodium nitrite and practically no weight
loss was measured. It also produces nitric oxide/dioxide upon addition of acids and appears to be exactly the same no matter how long heat is applied.
-The manipulator
We are all here on earth to help others; what on earth the others are here for I don't know. -W. H. Auden
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
MM here's the problem (from the paper cited earlier):

Look at the pressures in the final column, you need to do this under decent vacuum or continuous flow of an inert gas, the pressures are tiny and
you'd probably need to go in excess of 1000K.
That probably makes recapturing the decomposition gases impractical, so there goes that idea 
Full paper available online from: http://www.nist.gov/data/PDFfiles/jpcrd11.pdf
[Edited on 1-3-2015 by deltaH]
|
|
|
deltaH
Dangerous source of unreferenced speculation
    
Posts: 1663
Registered: 30-9-2013
Location: South Africa
Member Is Offline
Mood: Heavily protonated
|
|
I've taken the liberty of plotting those data points in the table against a logarithmic y-axis. Extrapolating visually, it looks like the pressure
reaches atmospheric somewhere around 1200-1400K (probably closer to 1400K to be safe) 
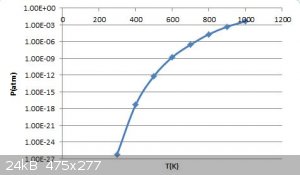
... perhaps a curve fit is in order.
[Edited on 1-3-2015 by deltaH]
|
|
|
xfusion44
Hazard to Others
  
Posts: 223
Registered: 6-8-2014
Location: Europe
Member Is Offline
Mood: Nostalgic
|
|
@Zyklon-A
Well, obviously I wasn't shocked... Like any previous gas, that ran through a plastic tube, that was used for this experiment (NO2, Cl2), the SO3 only
slightly decolored it. There was no explosions, vigorous reactions with water, "eating through plastic" and stuff like that. And I'm pretty sure, that
experiment scale matters a lot. Imagine a very small firecracker, detonating in your hand - there will be no, or almost no damage, but if the same is
done with a large firecracker, your hand might not be attached to your arm anymore. And yes, I believe you and others about what you say for SO3 gas.
Anytime I'm about to do an experiment, I always check on the internet and youtube, what might happen and what are the dangers and I know that when
dealing with pure SO3, even multiple protective gloves might not be enough, to stop it, but the scale of my experiment was just neglible. Few ml's of
gaseous SO3 can't just "burn" through the pipe...
|
|
|
Zyklon-A
International Hazard
    
Posts: 1547
Registered: 26-11-2013
Member Is Offline
Mood: Fluorine radical
|
|
Yes well the difference is the fact the it was gaseous. If it didn't damage the tube much, it's because either its more resistant than I thought
(unlikely) or practically no sulfur trioxide was formed (more likely). You see any and of the SO3 reacted and oxidized the tube. You just
didn't make much of it. The density of gasses is very small compared to solids and liquids.
Same explanation goes for the lack of explosion regarding sulfur trioxide and water. I doubt any sulfur trioxide even came in contact with liquid
water as it would have reacted with the tube and the water in the air first.
|
|
|
xfusion44
Hazard to Others
  
Posts: 223
Registered: 6-8-2014
Location: Europe
Member Is Offline
Mood: Nostalgic
|
|
Quote: Originally posted by Zyklon-A  | Yes well the difference is the fact the it was gaseous. If it didn't damage the tube much, it's because either its more resistant than I thought
(unlikely) or practically no sulfur trioxide was formed (more likely). You see any and of the SO3 reacted and oxidized the tube. You just
didn't make much of it. The density of gasses is very small compared to solids and liquids.
Same explanation goes for the lack of explosion regarding sulfur trioxide and water. I doubt any sulfur trioxide even came in contact with liquid
water as it would have reacted with the tube and the water in the air first. |
I'd say that it was formed (CuSO4 decomposed and test of water to which it was bubbled, showed that the pH was reasonably lowered). Although the
quantity was very small - only a gram or two of CuSO4 was used. I'm not sure about what the tubing was made of. I think it was PVC or PU - I'm not
sure for the PU, but PVC should be resistant to SO3, according to this: http://kuriyama.thomasnet.com/Asset/Tigerflex-2011-Catalog-F...
|
|
|
Zyklon-A
International Hazard
    
Posts: 1547
Registered: 26-11-2013
Member Is Offline
Mood: Fluorine radical
|
|
I must say I'm very suprised PVC will hold up to sulfur VI oxide, interesting.
When you say "only a gram or two was used" do you mean a gram of SO3 was evolved or a gram of copper sulfate was in the tube to start with?
|
|
|
woelen
Super Administrator
        
Posts: 7977
Registered: 20-8-2005
Location: Netherlands
Member Is Offline
Mood: interested
|
|
I doubt whether you get any SO3 from CuSO4. I expect formation of CuO, lower sulfur compounds of Cu, S and O, and gaseous SO2 with only traces of SO3.
I tried heating CuSO4.5H2O at very high heat and I always was surprised to see that I ended up with some brown material. First it becomes very pale
blue (dehydration), nearly white. Next, it turns a brownish color. I never investigated its precise properties, but it is insoluble in water. I also
smelled SO2.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Historically pyrolysis of 'green vitriol' - ferrous sulphate, was used to produce sulphuric acid. Anyone looking at preparing
SO<sub>3</sub> might look into that, rather than CuSO<sub>4</sub> pyrolysis.
[Edited on 2-3-2015 by blogfast25]
|
|
|
Magpie
lab constructor
    
Posts: 5939
Registered: 1-11-2003
Location: USA
Member Is Offline
Mood: Chemistry: the subtle science.
|
|
Quote: Originally posted by blogfast25  | Historically pyrolysis of 'green vitriol' - ferrous sulphate, was used to produce sulphuric acid. Anyone looking at preparing
SO<sub>3</sub> might look into that, rather than CuSO<sub>4</sub> pyrolysis.
[Edited on 2-3-2015 by blogfast25] |
eg:
"Until this process was made obsolete by the contact process, oleum had to be obtained through indirect methods. Historically, the biggest production
of oleum came from the distillation of iron sulfates at Nordhausen, from which the historical name Nordhausen sulfuric acid is derived."
-Wiki
The single most important condition for a successful synthesis is good mixing - Nicodem
|
|
|