NexusDNA
Hazard to Others
  
Posts: 104
Registered: 23-11-2013
Location: Brazil, under an umbrella
Member Is Offline
Mood: Liberated from cocoon
|
|
Help in problem
Reference: Mechanism and theory in organic chemistry, 1976, by Thomas Lowry and Kathleen Richardson.
9th chapter: "Radical reactions", problems section, 1st problem:
1) Propose a pathway to account for the formation of epoxides in autoxidation of olefins.
So I couldn't figure it out... I did understand that by reaction with peroxyacids alkenes can be converted to epoxides by the "butterfly mechanism" or
more formally the Prilezhaev reaction, but I'm not sure if peracids could be considered in the context of autoxidation.
What would you propose?
Bromine, definitely bromine.
|
|
|
Pasrules
Hazard to Self
 
Posts: 78
Registered: 4-1-2015
Location: Yellow Cake Deposit
Member Is Offline
Mood: Lacking an S orbital
|
|
This is a thread of mine with a proposal to oxidise an alkene (olefin). You should find some information on Epoxides posted by deltaH towards the end
https://www.sciencemadness.org/whisper/viewthread.php?tid=59...
As posted by deltaH Vicinal Diols: https://www.sciencemadness.org/whisper/files.php?pid=385286&...
[Edited on 11-3-2015 by Pasrules]
Atropine, Bicarb, Calcium.
|
|
|
NexusDNA
Hazard to Others
  
Posts: 104
Registered: 23-11-2013
Location: Brazil, under an umbrella
Member Is Offline
Mood: Liberated from cocoon
|
|
Yes, this is the peroxyacid (in this case performic) epoxidation, followed by hydration to make the diol.
Bromine, definitely bromine.
|
|
|
Darkstar
Hazard to Others
  
Posts: 279
Registered: 23-11-2014
Member Is Offline
Mood: Sleepy
|
|
@ Pasrules:
The OP is asking about autoxidation (i.e. a radical chain reaction between O2 and the alkene), not peroxy acids. The method deltaH was
referring to in your thread, where he suggested that you use hydrogen peroxide and formic acid, gives performic acid.
@ NexusDNA:
Yes, autoxidation of alkenes can produce epoxides. Unfortunately, the exact mechanism for these reactions isn't actually known, and there's likely
more than one mechanism responsible for epoxide formation anyway. With that said, I'd imagine the most probable mechanism(s) involves an intermediate
β-peroxyalkyl radical, with epoxidation proceeding through homolytic cleavage of the oxygen-oxygen single bond and subsequent reaction with the
adjacent radical electron.
I don't know how good you are with radical chemistry, so that may or may not help much. I'll try to draw up a quick mechanism for you in a little bit.
I'll post it if and when I do.
[Edited on 3-12-2015 by Darkstar]
|
|
|
Darkstar
Hazard to Others
  
Posts: 279
Registered: 23-11-2014
Member Is Offline
Mood: Sleepy
|
|
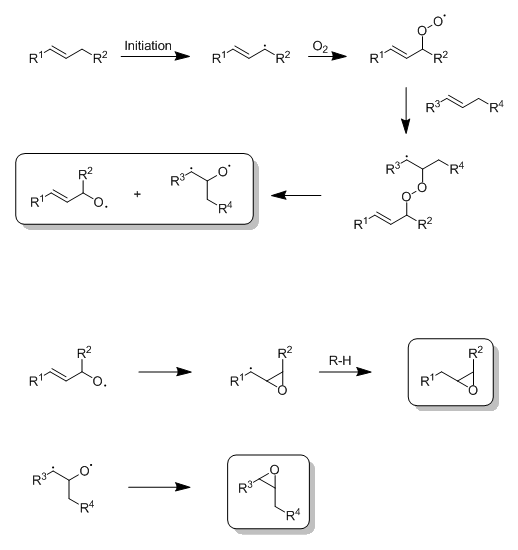
As promised, here's the pathway I proposed earlier. I didn't bother to show electron pushing, but it should be pretty straight forward. Autoxidation
is initiated via the removal of a hydrogen atom alpha to the double bond, generating an allylic radical. The radical then reacts with O2
and forms a peroxy radical, which then adds to the double bond of a second alkene molecule to give a β-peroxyalkyl radical. This β-peroxyalkyl
radical then fragments into two alkoxy radicals, which both might possibly undergo a ring closure to an epoxide; however, the bottom alkoxy radical is
probably the more likely candidate. Ring closure of the top alkoxy radical will probably depend a lot on just how well the adjacent R1 group can
stabilize the resulting radical carbon, as it still needs to abstract a proton.
|
|
|
NexusDNA
Hazard to Others
  
Posts: 104
Registered: 23-11-2013
Location: Brazil, under an umbrella
Member Is Offline
Mood: Liberated from cocoon
|
|
Wow! That's perfect! Thank you very much.  
I didn't make any progress because I was thinking only about ethylene and styrene. awesome!
What program did you use to draw?
Bromine, definitely bromine.
|
|
|
Darkstar
Hazard to Others
  
Posts: 279
Registered: 23-11-2014
Member Is Offline
Mood: Sleepy
|
|
No problem. Was this an actual homework problem of some kind, or was it just out of sheer curiosity? Glad I could help either way.
I used ChemDraw, by the way.
|
|
|
NexusDNA
Hazard to Others
  
Posts: 104
Registered: 23-11-2013
Location: Brazil, under an umbrella
Member Is Offline
Mood: Liberated from cocoon
|
|
I'm studying on my own! hehe, chemistry is beautiful. This was a problem in a textbook.
Bromine, definitely bromine.
|
|
|