| Pages:
1
2 |
Cou
National Hazard
   
Posts: 958
Registered: 16-5-2013
Member Is Offline
Mood: Mad Scientist
|
|
Game: Chemistry from natural resources only
When you are ordering pure chemicals from a site such as elemental scientific, and buying acetone or muriatic acid from a hardware store, it's easy to
forget that these things weren't made out of thin air; everything has to start off from natural sources. All the complicated organic medicines in the
pharmacy such as aspirin, they can be traced back to natural resources. My favorite example is a popular method of making phosphorus with access to a
very hot furnace:
Ca3(PO4)2+3 SiO2+5 C = 3 CaSiO3+5 CO+2 P
All those reagents can be obtained easily from the earth, even if you don't own a mineral mine. Calcium phosphate can be obtained by hunting animals
and grinding their bones into powder. Silicon dioxide is sand from the beach, it's what the earth is mostly made of; and carbon from decomposing sugar
at high temps or charcoal by burning wood.
Even your own body is a source of natural chemicals: You can extract urea and white phosphorus from your urine.
Unfortunately, many inorganic compounds are only found in specific minerals/ores which you must have access to a mine to get them. Iron is probably
the easiest metal to find in nature, in the form of iron oxide rocks. You also need crude oil to get alkanes.
I would like to start getting into natural resource chemistry, but I still need to learn some stuff. If you trace back the production of something
like aspirin (acetylsalicylic acid) all the way to harvesting of natural resources, what is the total synthesis from natural resources to aspirin?
And so here is the game we can play: How many chemicals can you trace back to their natural resources?
For example, let's do sulfuric acid (H2SO4).
In industry, sulfuric acid is made by dissolved sulfur trioxide is water. Sulfur trioxide is made by reacting sulfur dioxide with oxygen (natural
resource in the air). Sulfur dioxide is also made by reacting sulfur with oxygen. Sulfur comes from desulfurization of crude oil (natural resource)
with ethanethiol. Ethanethiol is made by reacting ethylene and hydrogen sulfide with aluminum oxide as a catalyst. Hydrogen sulfide is separated from
sour gas (a natural resource). Ethylene comes from steam cracking crude oil. Aluminum oxide is from the mineral corundum.
There, we have successfully traced the production of sulfuric acid back to natural resources: Oxygen, crude oil, corundum, sour gas.
Sulfur is also found around volcano vents, which makes amateur production of sulfuric acid from natural resources actually doable, if you live in
Hawaii, maybe.
And so if a chemophobic fool says "IT'S NOT A DANGEROUS CHEMICAL IF IT COMES FROM NATURE", just remember how many toxic things can be made from
nature.
[Edited on 11-4-2015 by Cou]
|
|
|
DraconicAcid
International Hazard
    
Posts: 4278
Registered: 1-2-2013
Location: The tiniest college campus ever....
Member Is Offline
Mood: Semi-victorious.
|
|
Aspirin's not that hard- you would extract salicylic acid from willow bark (it's present as some complicated ester, but can be hydrolyzed), and
acetylate it with acetic acid you've extracted from vinegar. On paper, at least.
Please remember: "Filtrate" is not a verb.
Write up your lab reports the way your instructor wants them, not the way your ex-instructor wants them.
|
|
|
unionised
International Hazard
    
Posts: 5102
Registered: 1-11-2003
Location: UK
Member Is Offline
Mood: No Mood
|
|
"Chemistry from natural resources only"
Where else would it come from?
|
|
|
Cou
National Hazard
   
Posts: 958
Registered: 16-5-2013
Member Is Offline
Mood: Mad Scientist
|
|

|
|
|
Fulmen
International Hazard
    
Posts: 1693
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
Unionised: The point is to do chemistry "from scratch", using only natural, unprocessed raw materials. I love this kind of work, partly "because I
can" but also because I love the history of science and technology. I have a dream of making a few grams of uranium and thorium from mines not too far
from me. I want to dig the rock out of the ground myself and end up with a cube of metal. Perhaps a retirement-project since the radiation won't be
much of an issue anymore 
We're not banging rocks together here. We know how to put a man back together.
|
|
|
Cou
National Hazard
   
Posts: 958
Registered: 16-5-2013
Member Is Offline
Mood: Mad Scientist
|
|
Quote: Originally posted by Fulmen  | Unionised: The point is to do chemistry "from scratch", using only natural, unprocessed raw materials. I love this kind of work, partly "because I
can" but also because I love the history of science and technology. I have a dream of making a few grams of uranium and thorium from mines not too far
from me. I want to dig the rock out of the ground myself and end up with a cube of metal. Perhaps a retirement-project since the radiation won't be
much of an issue anymore  |
I don't know how you would get access to a mine. Perhaps paying the owner for permission to enter for a day?
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Unionised nailed it : All chemicals are from natural sources.
The idea is intriguing though.
How to make X from the planet on which we stand (without just buying things).
Could be a good competition, e.g. make compound X using ONLY the dirt in your backyard, and the stuff that is living in it.
|
|
|
Cou
National Hazard
   
Posts: 958
Registered: 16-5-2013
Member Is Offline
Mood: Mad Scientist
|
|
Hydrochloric acid is made commercially by bubbling HCl gas through water. HCl gas is made by reacting chlorine and hydrogen gas with UV light, which
are both made by electrolysis of a saturated NaCl solution. So this is also doable if you can evaporate seawater.
|
|
|
Cou
National Hazard
   
Posts: 958
Registered: 16-5-2013
Member Is Offline
Mood: Mad Scientist
|
|
Hydrochloric acid is made commercially by bubbling HCl gas through water. HCl gas is made by reacting chlorine and hydrogen gas with UV light, which
are both made by electrolysis of a saturated NaCl solution. So this is also doable if you can evaporate seawater.
|
|
|
Etaoin Shrdlu
National Hazard
   
Posts: 724
Registered: 25-12-2013
Location: Wisconsin
Member Is Offline
Mood: Insufferable
|
|
Quote: Originally posted by the very first sentence in this thread  | | When you are ordering pure chemicals from a site such as elemental scientific, and buying acetone or muriatic acid from a hardware store, it's easy to forget that these things weren't made out of thin air; everything has to start off from natural
sources. |
|
|
|
Fulmen
International Hazard
    
Posts: 1693
Registered: 24-9-2005
Member Is Offline
Mood: Bored
|
|
There is a practical limit as to how far this can be taken, for instance you can't start mining iron just because you need a spring or make the lab
and the glassware from scratch. Making chemicals completely composed from base raw materials is in it self quite possible. Quite a few chemicals are
made mostly from air and water, but sooner or later you'll hit a logistical limit where the manufacture of reagents becomes far more arduous than the
"core" reaction.
We're not banging rocks together here. We know how to put a man back together.
|
|
|
Cou
National Hazard
   
Posts: 958
Registered: 16-5-2013
Member Is Offline
Mood: Mad Scientist
|
|
OK, here's the final chart for sulfuric acid (correct me if something is wrong with it):

[Edited on 12-4-2015 by Cou]
[Edited on 12-4-2015 by Cou]
|
|
|
j_sum1
Administrator
       
Posts: 6219
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
I wad hoping this was some kind of challenge or project.
Disappointed.
|
|
|
hyfalcon
International Hazard
    
Posts: 1003
Registered: 29-3-2012
Member Is Offline
Mood: No Mood
|
|
Take the knowledge and make your own project if you have an interest. Don't wait for someone else's idea.
|
|
|
Cou
National Hazard
   
Posts: 958
Registered: 16-5-2013
Member Is Offline
Mood: Mad Scientist
|
|
hyfalcon and j_sum1, I'm limited in my ability to do projects right now, because of lack of equipment. I won't be able to do much until my school's
chemistry club gets started.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
I still like this idea.
Basically Bootstrap from the same position humans were in a few hundred years ago, yet armed with all the knowledge gained since then.
Quote: Originally posted by Fulmen  | | you can't start mining iron just because you need a spring or make the lab and the glassware from scratch. |
Yes, you can.
Nothing stopping anyone doing any of that (all of which mostly involves Digging, Wood and Fire).
Arduous, vastly time-consuming, yet do-able.
Treat this as a pre-competition discussion.
Someone pick a reasonable target substance based on what is actually available in dirt and plants etc.
Essentials that were always available to all modern humans : sunlight, wood, water, wood, dirt, wood and Food of all kinds, and wood.
Uranium, Tantalum etc aren't universally available for some reason.
Internet can be treated as 'the accumulated knowledge' so is allowed.
|
|
|
bolbol
Hazard to Others
  
Posts: 167
Registered: 3-1-2015
Member Is Offline
Mood: No Mood
|
|
Id be interested to see Nitric acids diagram made from natural resources.
|
|
|
BromicAcid
International Hazard
    
Posts: 3227
Registered: 13-7-2003
Location: Wisconsin
Member Is Offline
Mood: Rock n' Roll
|
|
People that have been around the forum for some time probably remember:
http://www.cavemanchemistry.com/
Supposedly it covers exactly what you are looking for, staring from natural sources and how to get to final products from there. I believe the author
frequented this site for awhile as well. The table of contents can be viewed at the relevant website under the sample area. Here is a blurb from
the Amazon page:
| Quote: | | Half a million years ago our ancestors learned to make fire from scratch. They crafted intricate tools from stone and brewed mind-altering elixirs
from honey. Their descendants transformed clay into pottery, wool into clothing, and ashes into cleansers. In ceramic crucibles they won metal from
rock, the metals lead to colored glazes and glass. Buildings of brick and mortar enshrined books of parchment and paper. Kings and queens demanded
ever more colorful clothing and accessories in order to out-class clod-hoppers and call-girls. Kingdoms rose and fell by the power of saltpeter,
sulfur, and charcoal. And the demands of everyday folk for glass and paper and soap stimulated the first round of chemical industrialization. From
sulfuric acid to sodium carbonate. From aniline dyes to analgesic drugs. From blasting powder to fertilizers and plastics. In a phrase, From Caveman
to Chemist. |
| Quote: | | Calcium phosphate can be obtained by hunting animals and grinding their bones into powder. |
As someone who has gone that route... messy... messy... messy. Even buying pre-made bone meal there are a lot of fats and oils and garbage that hang
around. I tried to go the wet route to phosphoric acid and it was not nice. You really need to calcinate the bones first (i,e, make bone ash) to
drive off the organic nonsense before you would even consider this calcium phosphate.
[Edited on 4/14/2015 by BromicAcid]
|
|
|
Milan
Harmless

Posts: 30
Registered: 14-3-2015
Location: Europe
Member Is Offline
Mood: No Mood
|
|
Ok, here's the diagram for Nitric acid. 
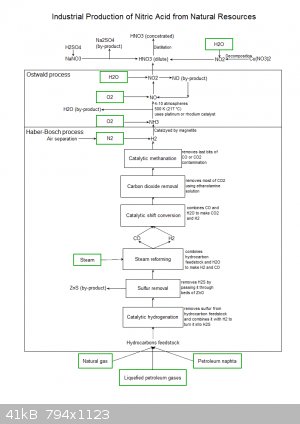
|
|
|
Bezaleel
Hazard to Others
  
Posts: 444
Registered: 28-2-2009
Member Is Offline
Mood: transitional
|
|
Well, that requires quite some specials.
Where would you get the required ethanolamine from? And from which natural resources will you make the catalysts for hydrogenation, shift conversion,
and methanation?
I much like the idea of this thread, although for my own experiments, I prefer to start from the (usually technical grade) stuff I find in hardware
and pottery supply stores.
|
|
|
The Volatile Chemist
International Hazard
    
Posts: 1981
Registered: 22-3-2014
Location: 'Stil' in the lab...
Member Is Offline
Mood: Copious
|
|
I prefer the same. But I also like mineral grade (i.e. iron from hematite, etc.). But the idea of making everything 'from scratch is kinda
ridiculously tough, why not add a limit on the glassware you're capable of making 
|
|
|
j_sum1
Administrator
       
Posts: 6219
Registered: 4-10-2014
Location: Unmoved
Member Is Offline
Mood: Organised
|
|
I guess the viability of this as a project depends on the nature of the target product.
Somewhere on the spectrum between essential oils and doped single crystal silicon wafers is a project waiting to happen. For me, I still haven't given
up on ntric acid from soy beans.
An iron nail is probably out of reach due to temperature requirements. I don't have a place to build a furnace. But something of that complexity could
be really fun.
|
|
|
Cou
National Hazard
   
Posts: 958
Registered: 16-5-2013
Member Is Offline
Mood: Mad Scientist
|
|
Quote: Originally posted by BromicAcid  | People that have been around the forum for some time probably remember:
http://www.cavemanchemistry.com/
Supposedly it covers exactly what you are looking for, staring from natural sources and how to get to final products from there. I believe the author
frequented this site for awhile as well. The table of contents can be viewed at the relevant website under the sample area. Here is a blurb from
the Amazon page:
| Quote: | | Half a million years ago our ancestors learned to make fire from scratch. They crafted intricate tools from stone and brewed mind-altering elixirs
from honey. Their descendants transformed clay into pottery, wool into clothing, and ashes into cleansers. In ceramic crucibles they won metal from
rock, the metals lead to colored glazes and glass. Buildings of brick and mortar enshrined books of parchment and paper. Kings and queens demanded
ever more colorful clothing and accessories in order to out-class clod-hoppers and call-girls. Kingdoms rose and fell by the power of saltpeter,
sulfur, and charcoal. And the demands of everyday folk for glass and paper and soap stimulated the first round of chemical industrialization. From
sulfuric acid to sodium carbonate. From aniline dyes to analgesic drugs. From blasting powder to fertilizers and plastics. In a phrase, From Caveman
to Chemist. |
| Quote: | | Calcium phosphate can be obtained by hunting animals and grinding their bones into powder. |
As someone who has gone that route... messy... messy... messy. Even buying pre-made bone meal there are a lot of fats and oils and garbage that hang
around. I tried to go the wet route to phosphoric acid and it was not nice. You really need to calcinate the bones first (i,e, make bone ash) to
drive off the organic nonsense before you would even consider this calcium phosphate.
[Edited on 4/14/2015 by BromicAcid] |
So to calcinate bones, heat them in a coal furnace at 1000-1500 C?
|
|
|
agent_entropy
Hazard to Self
 
Posts: 91
Registered: 17-7-2006
Location: U.S.
Member Is Offline
Mood: No Mood
|
|
I like this idea/concept/game. Actually it reminds me of the process of getting started in a game of Minecraft.
In fact, this kind of analysis is very useful because it shows what raw materials are strategic for maintaining access to a given compound.
|
|
|
HgDinis25
Hazard to Others
  
Posts: 439
Registered: 14-3-2014
Location: Portugal
Member Is Offline
Mood: Who drank my mercury?
|
|
I would rather think about it as an Amateur Chemistry Apocalypse where everything with a "chemical name" gets banned.
Salt can't be banned. One can electrolyze a solution of salt to produce Sodium Hydroxide and Chlorine.
Chlorine and Sodium Hydroxide makes Bleach. Chlorine and Water makes a mixture of Hypochlorous Acid and Hydrochloric Acid.
Sand and Sodium Hydroxide makes pure Silicon Dioxide:
https://hobbychemistry.wordpress.com/2015/04/11/pure-silicon...
Fermentation of Sugar leads to Ethanol.
Ethanol and Bleach makes Chloroform.
Citric Acid can be extracted from lemon juice.
Acetic Acid can be extracted from Vinegar.
This is just from the top of my head.
|
|
|
| Pages:
1
2 |