chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
Lead Tin Bismuth Alloys
I've been trying to find the optimal mixture of lead tin bismuth alloys that would have the lowest melting point.
Is anyone in possession of charts and such that give melting curves?
I know that Sn 60% and Pb 40% melts at 260 deg C (Wiki). Is anyone aware of what amount of Bi is required to lower the MP even further?
Also, when casting Sn/Pb mixes, it tarnishes from a beautiful metallic lustre to a dull grey. I tried reducing this with varnish, but in vain, the O2
seems to diffuse through. I'd want to avoid layering it with >1mm varnish.
Are there any tricks that make the allow more noble, i.e. by including trace elements, or varying the element ratios, or including more Bi?
PS I'd rather avoid exotic metals, including Ag, In, Cd etc. The idea is to cast metal at lowest MP possible that does not tarnish, containing as much
Pb/Sn as possible as they are comparably cheap.
PS2 SnCu0.7, with melting point of 227 °C, seems good, but I bet it tarnishes with such a high Sn content, plus it's going to be tough dissolving the
high MP Cu. Plus, I'd rather go for an alloy containing much lead as it is easier to obtain than Sn. Also, more Pb makes the alloy more visually
appealing, tin looks a bit boring.
[Edited on 14-7-2006 by chemoleo]
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
Nerro
National Hazard
   
Posts: 596
Registered: 29-9-2004
Location: Netherlands
Member Is Offline
Mood: Whatever...
|
|
Wat kind of varnish did you use? Maybe there are special metal varnishes?
And as for that eutectic, doesn't you universities library have charts of those? Triple metal eutectics must be 3D graphs, I must be honest and tell
you I havent seen those yet...
#261501 +(11351)- [X]
the \"bishop\" came to our church today
he was a fucken impostor
never once moved diagonally
courtesy of bash
|
|
|
chromium
Hazard to Others
  
Posts: 284
Registered: 27-6-2005
Member Is Offline
Mood: reactive
|
|
I suppose that any amount of bismuth to something like 40% (or maybe even 60%) can be used. IIRC lowest melting points that can be obtained by
alloying bismuth and Sn or bismuth and Pb are below 140C. More than 50% of bismuth is needed for this.
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
Yes it'd be a 3D graph. I am not in need of those, certain depressions in that graph are fine by me, which simply correspond to a low MP.
I used normal clear varnish from home depot. Next time I'll check for protective metal varnishes, good point.
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
chromium
Hazard to Others
  
Posts: 284
Registered: 27-6-2005
Member Is Offline
Mood: reactive
|
|
http://en.wikipedia.org/wiki/Rose_metal
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
Yes, I know Bi makes for an attractive target- however it isn't exactly cheap at £20/kg. Particularly if you need 1l of it. I was more after using Bi
as a dopant, and hopefully as an agent that'd stop this oxidation problem.
| Quote: | * SnAgCu solders are used by two thirds of Japanese manufacturers for reflow and wave soldering, and by about 3/4 companies for hand soldering.
o SnAg3.0Cu0.5, tin with 3% silver and 0.5% copper, has a melting point of 217-220 °C and is predominantly used in Japan. It is the JEITA
recommended alloy for wave and reflow soldering, with alternatives SnCu for wave and SnAg and SnZnBi for reflow soldering.
o SnAg3.5Cu0.7 is another commonly used alloy, with melting point of 217-218 °C.
o SnAg3.5Cu0.9, with melting point of 217 °C, is determined by NIST to be truly eutectic.
o SnAg3.8Cu0.7, with melting point 217-218 °C, is preferred by the European IDEALS consortium for reflow soldering.
o SnAg3.8Cu0.7Sb0.25 is preferred by the European IDEALS consortium for wave soldering.
o SnAg3.9Cu0.6, with melting point 217-223 °C, is recommended by the US NEMI consortium for reflow soldering.
* SnCu0.7, with melting point of 227 °C, is a cheap alternative for wave soldering, recommended by the US NEMI consortium.
* SnZn9, with melting point of 199 °C, is a cheaper alloy but is prone to corrosion and oxidation.
* SnZn8Bi3, with melting point of 191-198 °C, is also prone to corrosion and oxidation due to its zinc content.
* SnSb5, tin with 5% of antimony, is the US plumbing industry standard. Its melting point is 232-240 °C. It displays good resistance to thermal
fatigue and good shear strength.
* SnAg2.5Cu0.8Sb0.5 melts at 217-225 °C and is patented by AIM alliance.
* SnIn8.0Ag3.5Bi0.5 melts at 197-208 °C and is patented by Matsushita/Panasonic.
* SnBi57Ag1 melts at 137-139 °C and is patented by Motorola.
* SnBi58 melts at 138 °C.
* SnIn52 melts at 118 °C and is suitable for the cases where low-temperature soldering is needed.
|
This is from Wiki, on lead-free solders, and very informative.
I was hoping to find something similar on lead-containing solders...
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
Nerro
National Hazard
   
Posts: 596
Registered: 29-9-2004
Location: Netherlands
Member Is Offline
Mood: Whatever...
|
|
Shame on you! 5 minutes of googling gave me this: The effect of Pb contamination on the solification of Sn-Bi solders.
It states that the eutectic for this mixture is found at 51.5% Bi-15.5%Sn-33%Pb with a melting point at 95.3C (+- 0.5C) The percentages are mass
fractions.
[Edited on Fri/Jul/2006 by Nerro]
#261501 +(11351)- [X]
the \"bishop\" came to our church today
he was a fucken impostor
never once moved diagonally
courtesy of bash
|
|
|
12AX7
Post Harlot
    
Posts: 4803
Registered: 8-3-2005
Location: oscillating
Member Is Offline
Mood: informative
|
|
Bleh... lead-free solder sucks nard. Much higher melting point than Sn63, which is wonderful to use.
Ya I don't think you're going to find something that magically stops it from corroding, chromium aside. Of course, chromium isn't soluble in lead or
tin (well maybe tin, at a good temp, I don't know that). Certainly not any "trace" quantity. You'll have to plate it.
Tim
|
|
|
NeutralIon
Harmless

Posts: 28
Registered: 19-6-2005
Member Is Offline
Mood: No Mood
|
|
Indium Corp. makes several low temperature Bi/Pb/Sn alloys, for example:
#38 52.5% Bi, 32% Pb, 15.5% Sn eutectic at 95°C
#39 52% Bi, 30% Pb, 18% Sn eutectic at 96°C
#41 50% Bi, 28% Pb, 22% Sn eutectic at 100°C
and if you want even lower:
#136 49% Bi, 18% Pb, 12% Sn, 21% In eutectic at 58°C
Knowledge is Good
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
| Quote: | #38 52.5% Bi, 32% Pb, 15.5% Sn eutectic at 95°C
#39 52% Bi, 30% Pb, 18% Sn eutectic at 96°C
#41 50% Bi, 28% Pb, 22% Sn eutectic at 100°C |
| Quote: | | Shame on you! 5 minutes of googling gave me this: The effect of Pb contamination on the solification of Sn-Bi solders. |
| Quote: | Originally posted by chemoleo
Yes, I know Bi makes for an attractive target- however it isn't exactly cheap at £20/kg. |
I'd rather try and keep the Bi low (up to 10% perhaps), due to price.
Realising that the corrosion with time cannot be changed by adding other components (except maybe an alloy containing 80% Bi) I guess I'll have to
make do with special metal varnishes, and thick layers of it 
Thus I might as well use as Sn/Pb in roughly equal proportions (actually I find Sn much easier to obtain, in the form of Sn plates), and add a certain
unknown (yet) but small percentage of Bi to lower the MP as much as possible.
I suppose a 3D graph of MPs of Sn/Pb/Bi alloys would be perfect for this, correlated against some unit of optical appeal (metallic lustre)...but one
can't have it all 
[Edited on 16-7-2006 by chemoleo]
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
This thread evaded my browsing somehow. This type of thing is a direct offshoot of the research I do, and the standard reference book we use should
give a reference for the Sn-Pb-Bi system, it may end up containing just what ternary compounds exist(the research I do), but should hopefully include
melting points. I shall check for you on monday.
|
|
|
Fleaker
International Hazard
    
Posts: 1252
Registered: 19-6-2005
Member Is Offline
Mood: nucleophilic
|
|
I would suggest adding silver as a dopant, silver is highly soluble in lead, tin, and bismuth (Parks process and fire assaying rely on that fact); at
sufficient quantities, it should retard oxidation. Bismuth is even more prone to oxidation (when liquid) as a pure metal or a high Bi alloy than lead
or tin combined. It also oxidizes to an extent at room temperature going from a rose color to a duller gray. I might also suggest cadmium metal to
improve corrosion resistance and depress the melting point. I know you want to avoid the Ag and Cd, but unless you're willing to lay it on thick  , I don't see any other way. , I don't see any other way.
I do not know what part of Germany you are in Chemoleo, but I will sell a pound of 4N5 bismuth for 13 USD or (much less than the ~$24 you quoted
earlier) perhaps trade for something interesting. I also have powdered silver but my cadmium I keep in ingot form for obvious reasons.
[Edited on 16-7-2006 by Fleaker]
|
|
|
neutrino
International Hazard
    
Posts: 1583
Registered: 20-8-2004
Location: USA
Member Is Offline
Mood: oscillating
|
|
As usual, I suggest eBay for all your bismuth needs. There is a seller, hallmarkmetals, who sells 4N bismuth for US $8.50/lb and ships worldwide. At
any given moment, some other sellers will also offer it for sale.
Where do you get 4N5 bismuth? I've searched for it and have yet to find it at a reasonable price.
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
Fleaker, thanks for the offer, but I do have a regular albeit expensive supplier.
From personal experience, pure Bi doesnt oxidise at all, and its metallic lustre is still there after 2 years of storage. That can't be said for Pb
(which loses its lustre within days), and Sn, which loses it within months. I never came across a shiny tin- plate, did you ?
Anyway, this is why I figured Bi as a dopant might be useful to prevent surface oxidation, and to keep the alloy shiny.
Cadmium...well it's bloody toxic. And I certainly do not know any OTC supplier.
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
I have bismuth that was lab surplus.... 20 years later...still nice and shiny.
As promised, but late, here is the mostly complete melting point phase diagram. Another ref was in the literature, but my uni did not have it. Ill
post what it is tomorrow, I forgot it in the lab today as I ducked out early when the post doc stated playing with uranium...
[Edited on 21-7-2006 by rogue chemist]
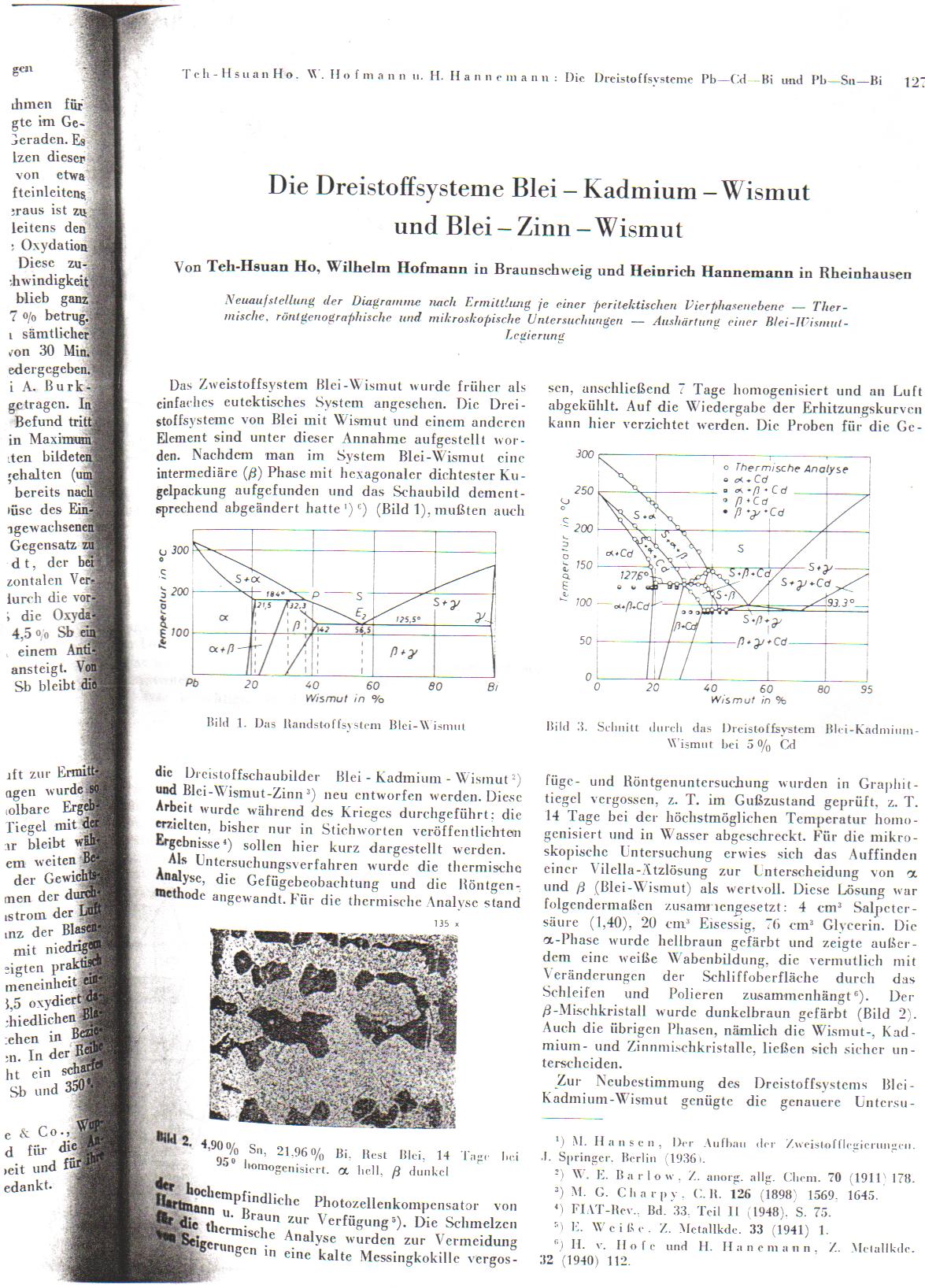
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
part 2....and a double post
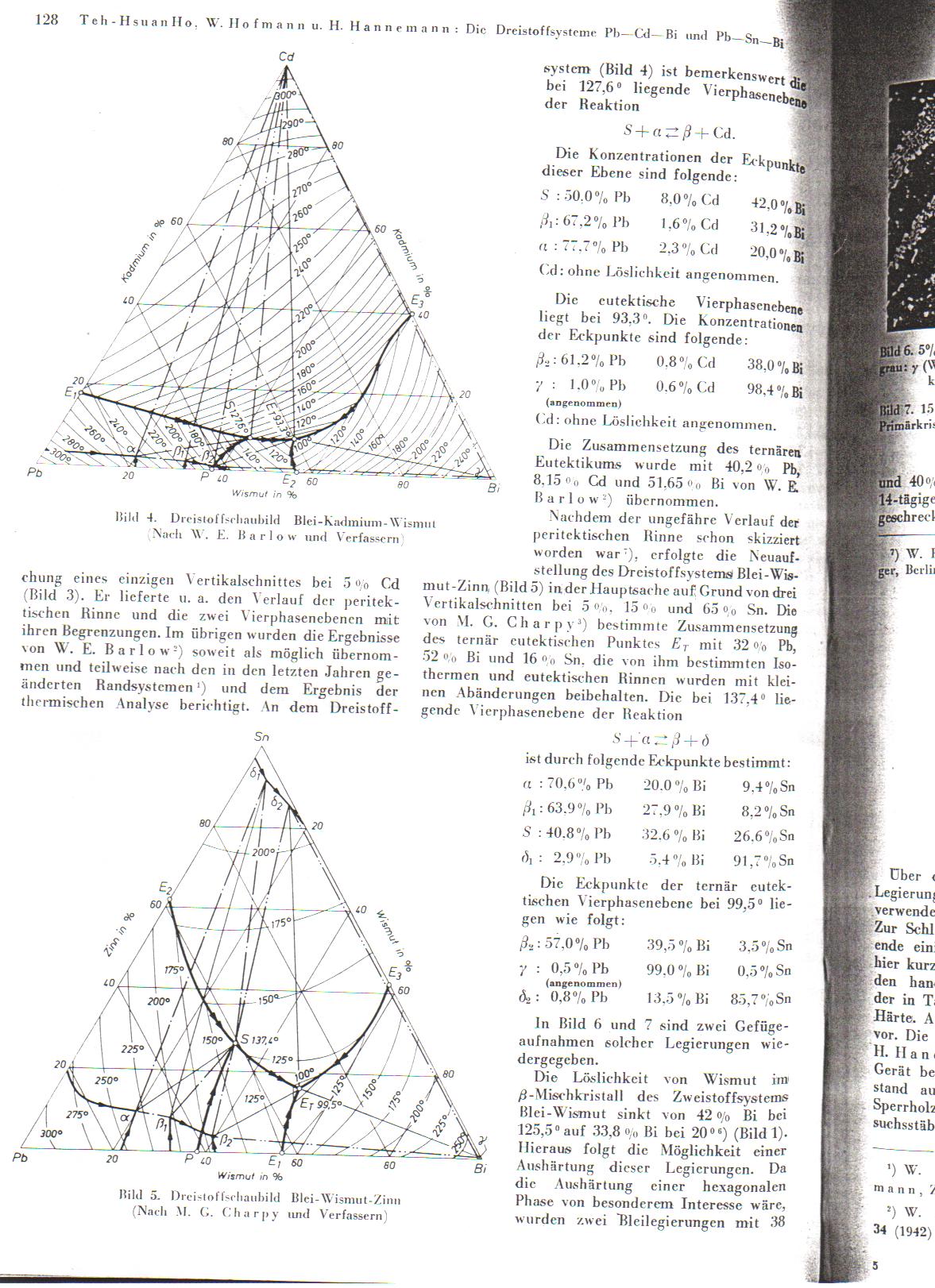
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
Triple post!
EDIT: I should mention, that I *think* that this is what you are looking for, my German is not so good anymore.
[Edited on 21-7-2006 by rogue chemist]
|
|
|
chemoleo
Biochemicus Energeticus
    
Posts: 3005
Registered: 23-7-2003
Location: England Germany
Member Is Offline
Mood: crystalline
|
|
Many many thanks Rogue.
I've posted the eutectic diagram of Sn/Pb/Bi alloys below:

I am, late as it is, not quite capable of interpreting it.
The german reference you posted says:
Temp readings (it doens't make that clear) are taken at 5, 15, and 65% Sn.
It also says, ' the corner points of the ternary euctectic 4-phase layer (direct translation) at 99.5 deg C are as follows:
Pb 57%, Bi 39.5 %, Sn 3.5% (not good because of high Bi)
Pb 0.5% Bi 99% Sn 0.5% (same)
Pb 0.8% Bi 13.5% Sn 85.7% (sounds most interesting). '
Also, seemingly melting at 137.4 deg C are
Pb 40.8 Bi 32.6 Sn 26.6
Pb 2.9 Bi 5.4 Sn 91.7
The last one sounds actually perfect... but I am not sure about hardness, metallic lustre etc.
Still I wondered, how are you to read this diagram? I can't work it out. Admittedly I had a few beers, so please if it is obvious spell it out for the
slow ones 
Thanks again Rogue!
PS the rest of the article refers to Cd, Bi/Pb mixes whcih i am not so interested in.
PS2 'Zinn' is tin, Sn, and 'wismut' is bismuth, Bi. Blei is lead, Pb.
[Edited on 21-7-2006 by chemoleo]
Never Stop to Begin, and Never Begin to Stop...
Tolerance is good. But not with the intolerant! (Wilhelm Busch)
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
Well, the percentages of the metals is the easiest part to read. The side of the triangle, for example, between the points labelled
Pb and Sn, has a 20, 40, 60, 80 scale, corresponding to the atomic(? it usually is in this field, but again I have seen both atomic and mass)
percentage. For example, that point 'S' is about 27% Sn, 35% Bi, and 40% Pb(pretty close to what they had there, big scale though). To calculate the
percentage based on a point in the ternary area(central area of triangle, anywhere that is not a direct line between 2 element corners) look to the
flat side of the triangle that is oposite the side with the desired element and follow the scale. Having the number scale in the side is more
annoying than helpful. Each line parallel to the side is 20% in this case.
I am not sure how to intrepet the temperature markings other than approaching the corners where it is obvious, the dotted and arrow lines mean nothing
to me.
A 4 phase area in the corners is unusual to my knowledge, usually only 3 phases, but what do I know, I am doing research heavy on crystalography,
never having taken a course in it yet...
PS: The name "Wismut" is awesome!
EDIT: PS: I understand German chemistry terms, its those annoying verbs and such that I do not know
[Edited on 21-7-2006 by rogue chemist]
[Edited on 21-7-2006 by rogue chemist]
|
|
|
praseodym
Hazard to Others
  
Posts: 137
Registered: 25-7-2005
Location: Schwarzschild Radius
Member Is Offline
Mood: crazy
|
|
But i guess we do have quite a few people here who can read german. Perhaps you can just post the original german copy here and someone will help in
the translation. Deutsch ist einfach!!   
Alles sollte so einfach wie möglich gemacht werden aber nicht einfacher.
|
|
|
chromium
Hazard to Others
  
Posts: 284
Registered: 27-6-2005
Member Is Offline
Mood: reactive
|
|
| Quote: | Originally posted by chemoleo
Cadmium...well it's bloody toxic. |
I may be wrong but i have got impression that cadmium is trully dangerous only if it is heated to partial vaporization - as in case of welding
(vapours ARE incredibly toxic) or ingested as soluble salts. At room temperature it does not seem more dangerous than lead and this may be so even at
temperatures that are used to make low melting alloys.
[Edited on 21-7-2006 by chromium]
|
|
|
The_Davster
A pnictogen
      
Posts: 2861
Registered: 18-11-2003
Member Is Offline
Mood: .
|
|
Praseodym, look 3,4 ,5 posts above yours. Ich habe diese Artikle geposted gestern.
|
|
|