| Pages:
1
2 |
DeusExMachina
Hazard to Others
  
Posts: 136
Registered: 14-10-2002
Location: pakistan
Member Is Offline
Mood: No Mood
|
|
Toxic or Poisoning Substances
I have been thinking about many different substances like sarin, VX gas and mustard gas or anyother poisoning or toxic thing. I have been wondering
how these substances work. I think most of you know my chemistry sucks and I am a slow learner  ... Please don't give me a whole bunch of chemical equations and stuff. I would like to know how these different things are formed or
what they do to affect someone's body. ... Please don't give me a whole bunch of chemical equations and stuff. I would like to know how these different things are formed or
what they do to affect someone's body.
Thanks!
and I don't think that this topic should only cover my question. I think other people should ask their questions that relate to this topic too.
|
|
|
Darkfire
Hazard to Others
  
Posts: 292
Registered: 3-1-2003
Location: California
Member Is Offline
Mood: Wondering
|
|
Hmm... Its hard to learn more advanced things when we cant use the basic words of science to show you...
Heres some good links that are for begginers.
http://faculty.washington.edu/chudler/weap.html
http://www.freebiehighway.com/survivalcenter/Cdefense/chemic...
CTR
|
|
|
DeusExMachina
Hazard to Others
  
Posts: 136
Registered: 14-10-2002
Location: pakistan
Member Is Offline
Mood: No Mood
|
|
no, you can use the scientific words and stuff. If I don't understand what the word means, I have a chemistry dictionary and if that doesn't
help, I'll use the regular dictionary.
|
|
|
DDTea
National Hazard
   
Posts: 940
Registered: 25-2-2003
Location: Freedomland
Member Is Offline
Mood: Degenerate
|
|
It's not so much that the nerve agents CAUSE the massive outpouring of Acetylcholine, which triggers muscle motion, but rather they block the
enzyme that breaks it down, Cholinesterase.
Therefore, as Acetylcholine is released by the automated nervous system, it triggers the muscles, and keeps triggering them since there is nothing to
stop it! It's basically like keeping your muscles in a constant "on" mode. Imagine your body becoming as stiff as a board, with your
back painfully arched.
Also, the automated nervous system controls Respiration and the Heart Beat... As such, when the muscles are constantly tensed, it becomes impossible
to breathe...death is usually from asphyxiation. However, in more rare instances, death can result from cardiac arrest.
|
|
|
Blind Angel
National Hazard
   
Posts: 845
Registered: 24-11-2002
Location: Québec
Member Is Offline
Mood: Meh!
|
|
I saw that tropine were use as a remedy, but is it only atropine or all Tropic acid like Scopolamin and other thing like that?
(eat some Datura Stramonium to get you butt saved...)
/}/_//|//) /-\\/|//¬/=/_
My PGP Key Fingerprint: D4EA A609 55E4 7ADD 8529 359D D6E2 33F6 4C76 78ED |
|
|
Darkfire
Hazard to Others
  
Posts: 292
Registered: 3-1-2003
Location: California
Member Is Offline
Mood: Wondering
|
|
There are several things used to treat NA's atropine is the most well known, im wondering how well cocaine would work, seeing how similar it is
to atropine.
CTR
|
|
|
Blind Angel
National Hazard
   
Posts: 845
Registered: 24-11-2002
Location: Québec
Member Is Offline
Mood: Meh!
|
|
yeah i'm looking at it but i think that it's the "Tropic Acid" part of the Atropine that do the job since most of these derivative
have the same effect. Cocaine doesn'T have the Tropic part. (Will post image later)
/}/_//|//) /-\\/|//¬/=/_
My PGP Key Fingerprint: D4EA A609 55E4 7ADD 8529 359D D6E2 33F6 4C76 78ED |
|
|
Blind Angel
National Hazard
   
Posts: 845
Registered: 24-11-2002
Location: Québec
Member Is Offline
Mood: Meh!
|
|
Here are the image, i did them a way so you can easily compare them.
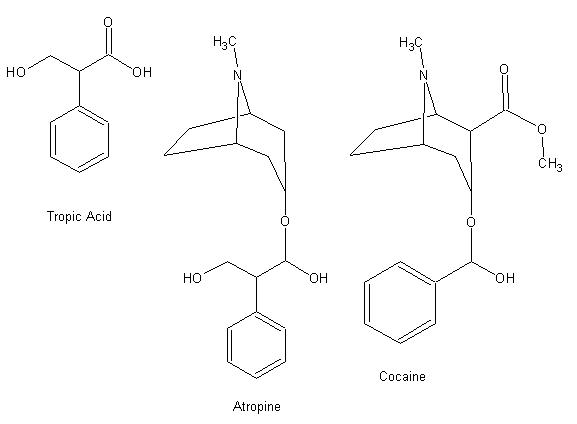
/}/_//|//) /-\\/|//¬/=/_
My PGP Key Fingerprint: D4EA A609 55E4 7ADD 8529 359D D6E2 33F6 4C76 78ED |
|
|
DDTea
National Hazard
   
Posts: 940
Registered: 25-2-2003
Location: Freedomland
Member Is Offline
Mood: Degenerate
|
|
I believe Atropine is preferred because of its potency and fast action, which is preferable to treat the potency and fast action of Nerve Agents. The
action of Atropine is literally the opposite of Nerve Agents...instead of locking the automated nervous system "on," it turns it
"off." As you know, "unregulated," or alone in the body, Atropine is itself a powerful poison. But, together with a Nerve Agent,
the effects of the two cancel each other out  . .
|
|
|
DeusExMachina
Hazard to Others
  
Posts: 136
Registered: 14-10-2002
Location: pakistan
Member Is Offline
Mood: No Mood
|
|
After wondering why I wasn't getting the answers I wanted, I figured that I completely asked the wrong question so I'm not blaming it on
anyone but myself.
The question should be: What happens in these substances that makes them harmful to the body? There has to be sometihng going on in the stuff to make
it so harmful to us.
|
|
|
Blind Angel
National Hazard
   
Posts: 845
Registered: 24-11-2002
Location: Québec
Member Is Offline
Mood: Meh!
|
|
I know that Mustard Gas simply turn into HCl in contact with the water of your skin (or any part of your body which has water in) so it's really
nasty. I know that alcool disolve fat around your brain cells (see my post Dumb Questions in Whimsy) other would need to be taken one by one...
Oh, and VX replace the Acetylcholine or inhibe the Cholinesterase so it can't unbound (some derivative of the Dextromerthorphane has the same
abilities). For mor info look for the Molecule of the Month page on ????.edu and select VX it's explained in details
/}/_//|//) /-\\/|//¬/=/_
My PGP Key Fingerprint: D4EA A609 55E4 7ADD 8529 359D D6E2 33F6 4C76 78ED |
|
|
DDTea
National Hazard
   
Posts: 940
Registered: 25-2-2003
Location: Freedomland
Member Is Offline
Mood: Degenerate
|
|
Ohh, you're interested in the chemical reactions that kill the target... Well, this will vary from agent to agent.
For any compound with a very reactive H in contact with water, they form HX (for Chlorine and Phosgene, this would be HCl). If I recall correctly,
this either collects in the lungs, or it corrodes the lining of the lungs. Either way, it causes a "chemical-pneumonia" which kills the
victim. This also explains the irritation of the mucous membranes caused by some of these compounds, especially Chlorine or Halo-benzene compounds.
Mustard Gas also kills the same way, if I recall correctly- through pulmonary edema/"Chemical Pneumonia" when inhaled. However, on contact
with skin, it interferes with Protein synthesis, causing its symbolic blistering.
I'm not sure about blistering agents such as Lewisite or Phosgene Oxime, however.
Nerve Agents work by bonding irreversibly to the active site on Cholinesterase. The active site on the Nerve Agent (For Sarin and Soman this active
site is F, for Tabun it is CN, for TEPP it is Diethyl Phosphate, etc...).
That's all I can remember right now, and all my facts may not be 100% accurate...this is because I am simply parroting what I have read over a
span of many months, so I can't even check all my sources  . .
|
|
|
Iv4
Hazard to Others
  
Posts: 312
Registered: 28-5-2003
Member Is Offline
Mood: No Mood
|
|
Atropine is used for quite a lot these days(though in very dilute concentrations).1% ointments can be picked up at the pet store.It's used for
dialating the pupils it's said to have a lethal dosage of 10mg.
It's said that it can produce erotic and pleasent dreams in low concentrations and nightmares in large ones.
|
|
|
Lugh
Hazard to Self
 
Posts: 66
Registered: 29-3-2003
Member Is Offline
Mood: No Mood
|
|
Atropine depresses the parasympathetic NS and excites the sympathetic NS, it is used in predoseing before surgery to reduce excretions from mucous
membranes like the saliva glands and the intestines. It is also used in eye surgry (dilates the pupil).
As far as i can remember the usual dosage for adults is about 0.02mg/kg. I cant remember what a fatal dose is.
10mg would be a dose for a person of 500kg\1100lb. More than enough!!!
Its can also be used to treat poisonong from Tabun, well in hamsters anyway! Can't rember the source but it was a tox study, i imagine it can
also be used in humans. That does not mean you should go and give yourself atropine if you one day happen to be playing with the big T and accidently
perform some rudimentry studies on your own person.
In that case you should sit in the corner and try to die with some dignity 
|
|
|
Blind Angel
National Hazard
   
Posts: 845
Registered: 24-11-2002
Location: Québec
Member Is Offline
Mood: Meh!
|
|
It is use in treatement of the intoxication on human, they were giving syringue with atropine in to soldier that had chance to come in contact with
nerve agent
/}/_//|//) /-\\/|//¬/=/_
My PGP Key Fingerprint: D4EA A609 55E4 7ADD 8529 359D D6E2 33F6 4C76 78ED |
|
|
gil
Unregistered
Posts: N/A
Registered: N/A
Member Is Offline
|
|
I "heard" strichinine is toxic too.I look fror the "Formula"
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
The nerve agents work exactly the same way that cockroack spray works. They inhibit an enzyme. The name of the enzyme is acetylcholine esterase
(AChE). Bug sprays are optimized to kill insects and not mammalian life, military nerve agents are optimized to kill higher forms of life, e.g.,
people. If you want to know why AChE is important in the body and exactly how these compounds inhibit it, you will need to learn a whole lot more
biochemistry and physiology.
Vesicants such as sulfur mustards etc. work by cross linking the two strands of DNA thus preventing DNA replication and repair. Once again, a deeper
insight would require that you understand DNA structure and the mechanism of its replication and repair, and the significance of the prevention of
that, at the genetic level, the molecular-bioilogical level.
Suffice it to say that you do not want to be anywhere around any of these things.
Watch a cockroach die after being sprayed and then ask yourself if you would care to be dying like that. Or check out the photos of what the mustards
do to human beings, big blisters, blindness, and if not a lethal instance, usually cancer later.
These are horrible substances and we all rather wish we could uninvent them. Every civilized nation has not moved to ban them and prevent anyone from
making or using them ever again.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
NO mustard does not work by hydrolyzing to HCl which would in no way explain its toxicity which is of a far higher degree than HCl.
The fact is that we did not understand the mechanism of mustard toxicity until Watson and Crick gave us the structure of DNA in the late 40s three
decades after the major use of mustard in warfare. Only then did we figure out how it worked.
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Atropine is obsolete as an antidote to GA and GB and was never effective against VX.
A later antidote although also passe now was 2-PAM, look it up.
2-pyridine aldoxime methiodide.
Now entirely different countermeasures are employed. These I will not discuss.
Atropine is an antagonist of nerve agents.
Cocaine blocks the reuptake of fopamine (a catecholamine) in the brain.
Similar structures with totally different pharmacology.
Atropine of course is a deadly poison unless you have been poisoned with a nerve agent. It is the principle alkaloid in belladonna, a popular poison
since the time of the Borgias. Or before.
|
|
|
vulture
Forum Gatekeeper
    
Posts: 3330
Registered: 25-5-2002
Location: France
Member Is Offline
Mood: No Mood
|
|
| Quote: |
These are horrible substances and we all rather wish we could uninvent them. |
I disagree RS-CH2-CH2-Cl reagents are needed as building blocks in the synthesis of sulfur heterocycles and thiacrownethers for example.
One shouldn't accept or resort to the mutilation of science to appease the mentally impaired.
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
@Sauron: Thanks for mentioning praldoxime (2-PAM, you beat me to it ). I was of
the impression, though, that it was most effective for undermining AChE inhibition caused by carbamates. In Tox. it was mentioned as less to
non-effective for hard-core organophophate esters like GB, etc. (for which atropine is, IIRC still applied). ). I was of
the impression, though, that it was most effective for undermining AChE inhibition caused by carbamates. In Tox. it was mentioned as less to
non-effective for hard-core organophophate esters like GB, etc. (for which atropine is, IIRC still applied).
Does anyone know the composition of US (or elsewhere) hip-jectors (I'd think a mixture of the two agents, additives, preservatives, etc.)?
For the record, overdosing on atropine is extremely easy (strammonium smokers beware of the seeds). They use physostigmine (a hard core carbamate AChE
inhibitor from Physostigma Venenosa) as an antidote.
Also, some vessicants, such as urushiol in poison ivy (and other toxicodendron species) work by binding (presumably via quinone to free protein NH2,
such as e-NH2-lysine) which then triggers an immune response. The susceptability to the toxin seems dependant upon the alkyl tail (where unsaturation
strongly favors vessicant activity). It seems to me that the tail is also acting a surfactant thereby disrupting cellular bilayers (besides serving as
an immunological target). Anyway, a classic, quite OTC vessicant which, as it appears to me could rather easily be engineered for *enhanced* activity.
I do not recall having ever read a paper where some (poor bastard) graduate student made and tested artificial urushiol analogues. Eew.
Just another bad idea,
O3
[Edited on 20-1-2007 by Ozone]
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
Thanks @ozone. I was referring to the classical military messicants, sulfur and nitrogen mustards, and the Lewisites. Obviously some other compounds
which produce blisters are "vessicants" without possessing the DNA cross-strand effect, the high systemic activity or military potential beyond maybe
harassment.
Cantharadins are toxic vessicants but I never heard of Spanish fly munitions.
Xylilidene dibromide is vessaicant but apart from having been tested as a potential weapon in WWI I never heard of it being weaponized. It produces a
feeling like intense sunburn.
I am just going by memory but 2-PAM was thought to be useful against GA/GB for a while. Not now.
Now I believe we mostly rely on prophylactic compounds (premedication of troops) which obviously is not done with atropine. There has been some
controversy about some of these compounds supposedly interacting with insect repellants but I have not paid much attention to the resolution of this
pissing contest.
As my interest in military organophosphates is entirely a peper exercise I do not need to be too concerned "personally" about these aspects, thank
God.
One of my buddies, Jack McGeorge, who was one of Hans Blix's UN inspectors in Iraq, just returned from a long sojourn there and lost about 10 Kg body
mass from sweating in his chemical protective gear. BTW he was the only UN inspector who actually FOUND anything (13 chemical rockets).
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
Are you referring to the prophylactic + DEET = Gulf War syndrome? Do you know what the prophylactic compound was?
I had to do some enzyme work once with diisopropylfluorophosphate, and my hands were shaking (which made it worse!). This is actually the surrogate
compound for the analytical method for GB (why bother with a surrogate that is *almost* as nasty as the analyte ?). ?).
Ethyl parathion is as hard core as I will get with the O/P's (since then). So I suppose..It's paper for me, too (unless I get called up on some
national security thing, then I'll gladly do it).
Tyvek in La. is bad enough...imagine MOPP gear in the desert. Booo!
Cheers,
O3
Are you sure Lewisite was a DNA "messicant" (I rather like that ). BAL could be
applied to ameliorate the effects, and IIRC it is a chelate. This implies that, unlike the mustards, it can be sequestered before permanent damage
occurs. If it was not so late I'd search. I must get some rest before qual. exam tomorrow...again...Booo! ). BAL could be
applied to ameliorate the effects, and IIRC it is a chelate. This implies that, unlike the mustards, it can be sequestered before permanent damage
occurs. If it was not so late I'd search. I must get some rest before qual. exam tomorrow...again...Booo!
I also like "fopamine", it could be a drug of abuse that explains much of what I observe from day to day ( I know what you meant, I just thought it funny). ( I know what you meant, I just thought it funny).
[Edited on 20-1-2007 by Ozone]
[Edited on 20-1-2007 by Ozone]
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
Sauron
International Hazard
    
Posts: 5351
Registered: 22-12-2006
Location: Barad-Dur, Mordor
Member Is Offline
Mood: metastable
|
|
@O3, I am pretty sure about L1/L2/L3 as they are structural analogs (2-chlorovinyl rather than 2-chloroethyl) but in both cases it's Cl-C-C-Z where Z
is a heteroatom S, N, or As. Se mustard is also toxic same way, Te is for some reason not particularly toxic (go figure.) Oh, there are oxygen
analogs as well. (bis(2-chloroethyl)ether is a carcinogen working just same way, the S mustards have the usual leg up because they slide through the
lipid barrier so easily.
The 2-chlorovinylthioethers are also deadly vessicants, those are precisely the sulfur analgs of the L series.
Given that the critical aspect of these molecules is the chlorine to chlorine distance in whatever the major molecular conformation might be, and the
relationship to the strand to strand distance in DNA, I'd be really surprised if the Lewisites did not share the mode of toxicity of the S and N
mustrads.
The Lewisites of course do have that extra fillip of systemic toxicity from the arsenic and I bet that is all that BAL addresses.
Remember any pre-Crick & Watson discussion of mechanism of any of these is rendered null and void.
Some compounds that are ALMOST in this class include sym-dichloromethyl ether, known carcinogen; USAF HA-5 which is analog of S-mistard in which Cl is
replaced by -CN; and the bromine analog and iodine analog.
|
|
|
Ozone
International Hazard
    
Posts: 1269
Registered: 28-7-2005
Location: Good Olde USA
Member Is Offline
Mood: Integrated
|
|
A little research yields...
This is sort of handy:
http://www.fas.org/nuke/guide/usa/doctrine/dod/fm8-9/3toc.ht...
They indicate that BAL is useful for ameliorating *some* of the symptoms latent from lewisite poisoning (they do not mention which ones).
However, it appears that (ding ding!) systemic As poisoning is a problem. It appears that lewisite inhibits pyruvate dehydrogenation (the As complexes
with lipoic acid) which derails energy production in the cell. Bummer.
I've read a several papers on this today and have found little reference to irreversible DNA implication (but nearly all still allude to-mechanism
unknown).
Sounds like BAL is quite unpleasant, but better than the alternative.
Structurally, the only other "popular" vesicant with a remotely "similar" Cl distribution is phosgen oxime. The mustards, both S and N all appear to
be multidentate ligands that do not likely act on the same target (or on the same target the same way) as either lewisite or phosgen oxime. Damn picky
biology!
Cheers,
O3
-Anyone who never made a mistake never tried anything new.
--Albert Einstein
|
|
|
| Pages:
1
2 |