Hawkguy
Hazard to Others
  
Posts: 326
Registered: 10-10-2014
Location: British Columbia (Canada eh!)
Member Is Offline
Mood: Body is Ready
|
|
Pentaerythritol - HOW does it work
Alright so after conquering a few recent chemistry projects, I'm planning for a Pentaerythritol synth. When looking for write-ups on the synthesis/
patents/ etc, I found three 'recipes'. Two involve the Para formaldehyde/ Acetaldehyde/ Calcium Hydroxide method, and another with some Sodium Formate
shit I potentially might have had to pay to read. I call these 'recipes' however because they don't actually explain the processes involved in the
synthesis (like what is actually going on) and that's half the fun of garage chemistry. It would be great if anyone can offer an explanation into the
details of the reaction (4 moles Para formaldehyde, 1 mole Acetaldehyde, 1 mole Calcium Hydroxide). Hopefully I made sense with this request, as I'm
only fumbling my way through high school chem.
|
|
|
hissingnoise
International Hazard
    
Posts: 3940
Registered: 26-12-2002
Member Is Offline
Mood: Pulverulescent!
|
|
In a nutshell, formaldehyde adds to the hydrogens of the methyl group forming 3 methylol groups, and a fourth CH2=O reacts with the
remaining CHO of acetaldehyde, forming the fourth methylol group with formic acid byproduct!
|
|
|
Hawkguy
Hazard to Others
  
Posts: 326
Registered: 10-10-2014
Location: British Columbia (Canada eh!)
Member Is Offline
Mood: Body is Ready
|
|
Thanks, much appreciated. I think I'm still going to probe the internet a bit more, but that answers a good deal once I can get around to
understanding it...
|
|
|
Dany
Hazard to Others
  
Posts: 482
Registered: 3-8-2013
Member Is Offline
Mood: No Mood
|
|
The following scheme is a representation of the formation of Pentaerythritol molecule from one acetaldehyde and 4 formaldehyde molecules in basic
media.
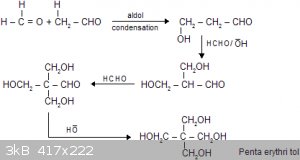
The reaction is catalyzed by bases such as calcium hydroxide-Ca(OH)2. Each time the hydroxide ion abstract a proton from the carbon
adjacent to the aldehyde function in the acetaldehyde which produces an enolate ion. The enolate ion being a nucleophile is able to attack a
formaldehyde molecule present in the solution. The reaction is repeated 3 times until no more hydrogen is left. The aldehyde function present in the
tri-hydroxylic compound undergoes a reaction called the Cannizzaro reaction where the aldehyde group (in the tri-hydroxilic compound) is reduces to
hydroxyl group and thus the formation of the Pentaerythritol (see last step) and at the same time a formaldehyde molecule is oxidized to formic acid
(which is the neutralized by the base present in the mixture).
Dany.
|
|
|
byko3y
National Hazard
   
Posts: 721
Registered: 16-3-2015
Member Is Offline
Mood: dooM
|
|
It's all about aldol condensation and cannizzaro. Aldehyde/ketone can add aldehyde as far as it has alpha-hydrogen. Acetaldehyde has 3 alpha
hydrogens, so it can add aldehyde 3 times. Of course it can add itself, leading to byproducts.
The last step, as far as i see, requires relatively strong basic conditions, leading to formic acid and pentaerythrol. You could ask "why formaldehyde
is oxidized and tris(metylol)acetaldehyde is reduced?". Well, because formaldehyde has a lot of hydrogens and that's why is a reducing agent, just
like formic acid.

[Edited on 18-6-2015 by byko3y]
|
|
|
Hawkguy
Hazard to Others
  
Posts: 326
Registered: 10-10-2014
Location: British Columbia (Canada eh!)
Member Is Offline
Mood: Body is Ready
|
|
Thanks that's what I needed.
|
|
|