| Pages:
1
2 |
uzaymaymunu
Harmless

Posts: 34
Registered: 26-2-2012
Location: turkey
Member Is Offline
Mood: No Mood
|
|
Metallic Strontium
Is it possible to get metallic strontium for us?
I mean:
A-With downs cell is possible?
B-Can someone explain the industrial method with details (Sr-oxide +Al) ?
C-Is it possible to get metallic Sr with catalyzed magnesium reduction method to get potassium.
Thanks.
uzaymaymunu.blogspot.com
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
A) Yes. Building an amateur Downs Cell, though, is quite a different matter.
B) Mix stoichiometrically. Set on fire. Collect metal.
C) What? Do you want Sr or K? Explain.
Request this thread to be moved to Beginnings.
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
Hawkguy
Hazard to Others
  
Posts: 326
Registered: 10-10-2014
Location: British Columbia (Canada eh!)
Member Is Offline
Mood: Body is Ready
|
|
Quote: Originally posted by uzaymaymunu  | Is it possible to get metallic strontium for us?
I mean:
A-With downs cell is possible?
B-Can someone explain the industrial method with details (Sr-oxide +Al) ?
C-Is it possible to get metallic Sr with catalyzed magnesium reduction method to get potassium.
Thanks. |
I am so confused. It doesn't help your case for the catalyzed Magnesium (reduction) method that
Strontium has a high melting point.
|
|
|
battoussai114
Hazard to Others
  
Posts: 235
Registered: 18-2-2015
Member Is Offline
Mood: Not bad.... Not bad.
|
|
Quote: Originally posted by elementcollector1  | A) Yes. Building an amateur Downs Cell, though, is quite a different matter.
B) Mix stoichiometrically. Set on fire. Collect metal.
C) What? Do you want Sr or K? Explain.
Request this thread to be moved to Beginnings. |
C)I think he meant similarly to what is done to get potassium,
|
|
|
violet sin
International Hazard
    
Posts: 1475
Registered: 2-9-2012
Location: Daydreaming of uraninite...
Member Is Offline
Mood: Good
|
|
" C-Is it possible to get metallic Sr with catalyzed magnesium reduction method to get potassium. "
I read this as:
C- Is it possible to get metallic Sr with ( a ) catalyzed magnesium reduction(, like that) used to get potassium.
|
|
|
elementcollector1
International Hazard
    
Posts: 2684
Registered: 28-12-2011
Location: The Known Universe
Member Is Offline
Mood: Molten
|
|
Oooh. Then, the answer is likely no - even if strontium did form, it'd probably form as a coating on the magnesium. Potassium works well because it
melts off.
Elements Collected:52/87
Latest Acquired: Cl
Next in Line: Nd
|
|
|
WGTR
National Hazard
   
Posts: 971
Registered: 29-9-2013
Location: Online
Member Is Offline
Mood: Outline
|
|
My first thought would be a modification of "A". I haven't tried this, so your mileage may vary (YMMV). If anyone thinks I'm overlooking something
obvious, jump on in.
Using a KCl-SrCl2 melt...
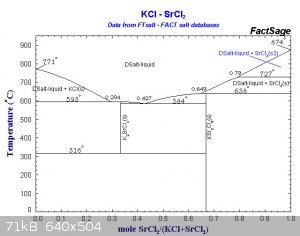
...electrolyse strontium into a molten copper/strontium cathode. The alloy should be kept concentrated enough in copper that it will stay underneath
the electrolyte. Two anodes would be used, one graphite, and the other made from copper. The current would be split between the two anodes to both
initiate and maintain the proper alloy in the cathode as more strontium is deposited. Here is the Cu-Sr system:
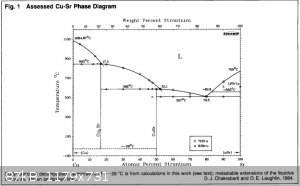 [3] [3]
Potassium and strontium are immiscible in both solid and liquid states [1]. Potassium and copper are also immiscible in both solid and liquid states
[2]. For this reason I don't think potassium would compete favourably with strontium for deposition, which is readily soluble in copper, and forms
stable compounds with it [3], at least as long as the strontium concentration doesn't go too high.
After batch electrolysis is accomplished, I think the potential could be reversed in the cell, with the molten cathode becoming the anode. The
strontium would be stripped from the anode (with some magnetic stirring of the molten anode probably required), and deposited as a solid at the
cathode. I don't know if potassium would co-deposit with it or not at this point, but chlorine would not be produced during this purification step.
1. http://dx.doi.org/10.1007/BF02869237
2. http://dx.doi.org/10.1007/BF02868995
3. http://dx.doi.org/10.1007/BF02872963
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Aluminothermic reduction of SrO with Al? That reduction is not thermodynamically favourable in open crucible conditions.
But in analogy with MgO/Al, Sr preparation by this method but under high vacuum and at high temperature (> 1,000 C) might be possible.
Sr's BP of 1,377 C is strongly lowered in high vacuum but remains higher than Al's.
The equilibrium 3 SrO(s) + 2 Al(l) < === > 3 Sr(g) + Al2O3(s) then shifts to the right because Sr vapour is distilled off.
Not for the faint of heart and certainly NOT Classic Thermite ('light and go')!!!
[Edited on 23-6-2015 by blogfast25]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Old fashioned but very doable on a lab/hobby level: electrolysis of Sr salt watery solution with a mercury cathode.
An Sr amalgam forms. Strip off mercury by vacuum distillation or under protective Ar blanket.
|
|
|
uzaymaymunu
Harmless

Posts: 34
Registered: 26-2-2012
Location: turkey
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by blogfast25  | Old fashioned but very doable on a lab/hobby level: electrolysis of Sr salt watery solution with a mercury cathode.
An Sr amalgam forms. Strip off mercury by vacuum distillation or under protective Ar blanket. |
Yes! sounds very good 
But I don't understand sth: Why it isn't the industrial method?
It looks like cheaper than that:
"The metal is produced commercially by reducing strontium oxide with aluminium. The strontium is distilled from the mixture."
Guys, I'm sorry about my poor english :/
[Edited on 24-6-2015 by uzaymaymunu]
uzaymaymunu.blogspot.com
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
Quote: Originally posted by blogfast25  | Old fashioned but very doable on a lab/hobby level: electrolysis of Sr salt watery solution with a mercury cathode.
An Sr amalgam forms. Strip off mercury by vacuum distillation or under protective Ar blanket. |
While this process is doable by amateurs, I'm not necessarily sure that it is safely doable by amateurs. Vacuum distillation of mercury is quite
difficult to perform safely in an amateur setting, especially considering the high density of mercury and thus the risk of condensing droplets
cracking the glass receiving vessel.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
That last mentioned risk is so easy to engineer against, it isn't even worth mentioning. Mercury may be dense but it's also a liquid and thus
extremely deformable.
Safety concerns are 'how long is a piece of string?' kind of issues. A bit like 'is it OTC?'. Let the experimenter decide, I say. Anyone who will
attempt to prepare metallic Sr has to be a decent chemist or forget about that altogether and that's true of most chemical endeavours worth doing.
Incidentally and not that it matters much but compared to WGTR's scheme, amalgamatic Sr from electrolysis is a walk in the park.
[Edited on 24-6-2015 by blogfast25]
|
|
|
Brain&Force
Hazard to Lanthanides
    
Posts: 1302
Registered: 13-11-2013
Location: UW-Madison
Member Is Offline
Mood: Incommensurately modulated
|
|
Quote: Originally posted by uzaymaymunu  | Is it possible to get metallic strontium for us?
I mean:
A-With downs cell is possible?
B-Can someone explain the industrial method with details (Sr-oxide +Al) ?
C-Is it possible to get metallic Sr with catalyzed magnesium reduction method to get potassium.
Thanks. |
I read this as "is it possible to *use metallic Sr with catalyzed magnesium reduction method to get potassium."
I don't see why not. But OP, please clarify what you mean.
At the end of the day, simulating atoms doesn't beat working with the real things...
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Really B&F? Considering how 'goldilocks' the reduction of KOH with Mg powder and t-butanol (assuming this is what the OP is referring to) actually
is, there's little reason for optimism to reduce Sr(OH)2 by that method.
|
|
|
phlogiston
International Hazard
    
Posts: 1375
Registered: 26-4-2008
Location: Neon Thorium Erbium Lanthanum Neodymium Sulphur
Member Is Offline
Mood: pyrophoric
|
|
One method I haven't seen mentioned yet is by thermal decomposition of strontium azide.
Doable on a lab scale, but involves toxic and explosive compounds. However, as blogfast25 remarked, any method to make Sr is going to require some
skill.
-----
"If a rocket goes up, who cares where it comes down, that's not my concern said Wernher von Braun" - Tom Lehrer |
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Heat sensodyne toothpaste to 900C and electrolyse, yielding a very small quantity indeed.
[Edited on 24-6-2015 by aga]
|
|
|
battoussai114
Hazard to Others
  
Posts: 235
Registered: 18-2-2015
Member Is Offline
Mood: Not bad.... Not bad.
|
|
Quote: Originally posted by aga  | Heat sensodyne toothpaste to 900C and electrolyse, yielding a very small quantity indeed.
[Edited on 24-6-2015 by aga] |
Could I chew some strontium for my sensitive teeth too?
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  | Heat sensodyne toothpaste to 900C and electrolyse, yielding a very small quantity indeed.
[Edited on 24-6-2015 by aga] |
Did you mean fluorine?
|
|
|
uzaymaymunu
Harmless

Posts: 34
Registered: 26-2-2012
Location: turkey
Member Is Offline
Mood: No Mood
|
|
Guys, I have master degree on organic chemistry. And I'm planning to do this experiment in fume hood.
Quote: Originally posted by Brain&Force  | Quote: Originally posted by uzaymaymunu  | Is it possible to get metallic strontium for us?
I mean:
A-With downs cell is possible?
B-Can someone explain the industrial method with details (Sr-oxide +Al) ?
C-Is it possible to get metallic Sr with catalyzed magnesium reduction method to get potassium.
Thanks. |
I read this as "is it possible to *use metallic Sr with catalyzed magnesium reduction method to get potassium."
I don't see why not. But OP, please clarify what you mean. |
No  I mean this: Sr(OH)2 + Mg I mean this: Sr(OH)2 + Mg
[Edited on 24-6-2015 by uzaymaymunu]
uzaymaymunu.blogspot.com
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
How, in which conditions?
|
|
|
battoussai114
Hazard to Others
  
Posts: 235
Registered: 18-2-2015
Member Is Offline
Mood: Not bad.... Not bad.
|
|
Nope, it's indeed strontium. They put Strontium chloride in toothpaste for sensitive teeth... If I had to guess its probably due to how the body
assimilates strontium in calcium formations (like bones) due to their similarities.
Ref for the use of strontium in toothpaste: http://www.webmd.com/vitamins-supplements/ingredientmono-107...
|
|
|
uzaymaymunu
Harmless

Posts: 34
Registered: 26-2-2012
Location: turkey
Member Is Offline
Mood: No Mood
|
|
I'm confused about something:
When you electrolize brine solution, the half reaction on cathode is: H+ + e- ----> H2
It's normal because reduction potential of sodium ion is -2.7 Volt.
So, I didn't understand why Sodium ions reduce on Castner-Kellner process?

[Edited on 26-6-2015 by uzaymaymunu]
[Edited on 26-6-2015 by uzaymaymunu]
[Edited on 26-6-2015 by uzaymaymunu]
uzaymaymunu.blogspot.com
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Thanks, Battou.
Quote: Originally posted by uzaymaymunu  | I'm confused about something:
When you electrolize brine solution, the half reaction on cathode is: H+ + e- ----> H2
It's normal because reduction potential of sodium ion is -2.7 Volt.
So, I didn't understand why Sodium ions reduce on Castner-Kellner process?

|
Only in the mercury cell is Na<sup>+</sup> reduced to Na (amalgam).
Not in the membrane process:
https://en.wikipedia.org/wiki/Chloralkali_process#Membrane_c...
It's a good question though and I haven't given it much thought up to now. I suspect it has to do with the overpotential for
H<sup>+</sup>/H<sub>2</sub> on mercury. Not sure though...
[Edited on 26-6-2015 by blogfast25]
|
|
|
uzaymaymunu
Harmless

Posts: 34
Registered: 26-2-2012
Location: turkey
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by blogfast25  | I suspect it has to do with the overpotential for H<sup>+</sup>/H<sub>2</sub> on mercury. Not sure though...
[Edited on 26-6-2015 by blogfast25] |
Why? I think only more than 2.7 V potential, Na+/Na reduction is possible. Under 2.7 V potential, only H+/H2 reduction is possible.
(I'm just thinking loudly, not sure)
uzaymaymunu.blogspot.com
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by uzaymaymunu  |
Why? I think only more than 2.7 V potential, Na+/Na reduction is possible. Under 2.7 V potential, only H+/H2 reduction is possible.
(I'm just thinking loudly, not sure) |
No. I don't think what you're saying is correct. But I wish someone else could throw some light on this.
Electrochemists of SM, unite!
|
|
|
| Pages:
1
2 |