| Pages:
1
2 |
quantumcorespacealchemyst
Banned Shitposter
Posts: 213
Registered: 17-10-2014
Member Is Offline
Mood: No Mood
|
|
PUZZLED UI3 (some UI4 maybe) synthesized, possibly, 238metal
BOLD letters for skimming, to the points if you don't wan't to read the details of synthesis
0.780g U-238 (milligram scale supposed +- 0.005g accuracy, confounding detail is one weight calibrater started rusting[extra weight] then i lost it
anyway, so trimmed a copper endcap to weigh 10.000g weighed side by side the original for a replacement, then recalibrated, but the swing seems
greater than +-0.005g now perhaps a 10mg swing sometimes)
(0.780[g]/238.05078826[g/mol])(3)(126.90447[g/mol]) = 1.247450017g Iodine for theoretical UI3 (i like using the full data even if my instruments are
not as good as them)
weighed out 2.202g i think; howver i think the double check read out as 2.165g, so this is where i believe i started questioning my scales functioning
more, as it was a significant figure of from the tech specs (it always floated around and this was alot worse).
having read the data that Uranium is pyrophoric, i did not want to attempt to powder it, and also i did not have a way (as far as i could imagine at
the time) to ensure against product loss let alone crush/grind the hard metal if it wasn't.
i put it in a tiny glass jar with the powdered Iodine and used teflon sheet to seal under the cap. it did not seem to react at all as say Arsenic
does; however, the arsenic takes a week to weeks to get to the point where it can be shook around to make a playdoh like blob over a few days. the
Uranium being solid, was probably going to take alot longer, if it ever would start dissolving off the skin layer (if it formed) and exposing fresh
metal.
i looked for info on how it may react without it, the iodine or the iodide formed reacting with the solvent.
i saw that UCl3 or possibly UCl4 dissolves in a polar aprotic solvent somewhere and it is frustrating that i am currently unable to find where that
data is again as science relies on wells of information. editors on wikipedia seem to get rid of good information in their ignorance of this.
i figured that UI3 would possibly do the same and read that Acetone is Polar Aprotic.
i did an experiment with i believe 10ml AcMe and the Uranium, Iodine mixture outdoors.
it effervesced slightly and i felt i warm slightly, although it may have been my hand holding it warming which i refelt after feeling it again after
my hand cooled down in the colder air.
after i was mostly confident it was not over-reacting, i set up for reflux and gradually raised the temperature.
eventually it was refluxing for about 3-5hours after which there was still metal in the vessel, which was able to be heard, after cooling and
septumed, by jerking it side to side a bit.
overnight, the metal seemed to have dissolved, as it did not make the tinking noise when jerked and shook it alittle.
if i remember correctly, tilting and rotating the vessel to move the viscous liquid, showed no sign of the metal left in place or sliding around. i
may be mistaken.
so the puzzle is that THERE WAS NO DETECTED RADIATION by my detector when held to the glass when in the jar and when dissolved (i believe the metal
chunk at the bottom immediatedly after reluxing didn't even set off the detector through the glass vessel)
;HOWEVER, after distilling the Acetone out
(found it distilled around 80-90ish C so it did not appear to have iodinated the AcMe as i wondered about and or catalyzed and/or reacted it as i
wondered about)
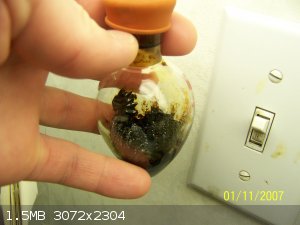
mostly to dryness (the tiny sludgeyness in the picture is the bit from the bottom, moving to the side as it was stored tilted that way in a lead
storage case)
THE SOLID that cakes the walls of the flask (and looks awesome, did not come through the best on the pictures) like scoria, where the bubbles of AcMe
burst from (presumed), IS RADIOACTIVE THROUGH THE GLASS.
solid metal with powdered iodine through jar = NON ACTIVE
solid metal in sludge of presumed iodide through flask = NON ACTIVE
presumed iodide solid through flask = ACTIVE
i was told "UI4 is easily decomposed to UI3."
[Edited on 29-6-2015 by quantumcorespacealchemyst]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
| Quote: | | 1.247450017g Iodine |
10 significant digits, as opposed to 4 for actual weighing. That’s silly, to put it mildly. It serves no purpose whatsoever, whatever you 'like it
'or not.
| Quote: | solid metal with powdered iodine through jar = NON ACTIVE
solid metal in sludge of presumed iodide through flask = NON ACTIVE
presumed iodide solid through flask = ACTIVE |
U238 is an alpha emitter, those don’t get through glass.
Unless iodine had been transmuted by alphas (very unlikely IMHO) your result is due to contamination (e.g. during distillation or refluxing) or
another measuring error. A bit of U238 (or U238 iodide) made it to the outside of the flask, onto your fingers or such like.
| Quote: | | i saw that UCl3 or possibly UCl4 dissolves in a polar aprotic solvent somewhere and it is frustrating that i am currently unable to find where that
data is again as science relies on wells of information. editors on wikipedia seem to get rid of good information in their ignorance of this.
|
Please don't blame Wikipedia for your own literature search incompetence.
[Edited on 29-6-2015 by blogfast25]
|
|
|
quantumcorespacealchemyst
Banned Shitposter
Posts: 213
Registered: 17-10-2014
Member Is Offline
Mood: No Mood
|
|
flask cleaned multiple times. if i clean it with acetone i may post another picture with the readings.
i thought of the possibility of that being the contamination source and washed it and washed it later again.
its been washed at least 2-3 times and i don't believe a tiny bit like that would set the detector off, seeing as i wiped the a bit from between the
neck and distillation adapter on paper and that hadn't set off the detector prior to drying. i assume any UI3 would be more heavily concentrated there
if present than the outside of the clear vessel.
|
|
|
Sulaiman
International Hazard
    
Posts: 3555
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
given the possibility of generation of radioactive iodine
you could test for this by checking for a half-life shorter than uranium ?
131 = beta decay so probably will not penetrate glass,
129 = beta + gamma but 1/2-life 15.7 Myears
125 is a gamma emitter, half life 13 hours, so that would be detectable
apparently there are 37 known isotopes of iodine https://en.wikipedia.org/wiki/Isotopes_of_iodine#Iodine-135
and my nuclear chemistry knowledge is way too limited for this task,
but if you set up a fixed test rig you may be able to detect some half-life activity.
it would be a nice/simple experiment.
P.S. when you hear the 'click' 'click' ... of a geiger counter you may assume that there are only a few particles per second emitted by radioactive
sources,
if you make or buy a spinthariscope https://en.wikipedia.org/wiki/Spinthariscope or e.g. http://www.ebay.co.uk/itm/NEW-Spinthariscope-experimenters-K...
you see thousands of decays per second (using a smoke detector Am source)
(I believe that in US it is illegal to take the Am source out of a smoke alarm)
I made my own using a CdS screen e.g. http://www.ebay.co.uk/itm/ZnS-Ag-over-Thin-Film-Mylar-2-dia-...
an eyepiece like this http://www.ebay.co.uk/itm/Glass-Watch-Repair-10X-Eye-Loupe-P...
and the Am source from a smoke alarm
.... beautiful to watch... a cheap but fascinating project.
The Am is theoretically an alpha emitter, so a polycarbonate or glass lens will protect your eye
(I found that there is actually quite a bit of gamma radiation from the Am decay chain products)
I would not use a gamma (or neutron) source for a spinthariscope due to the potential risk to eye tissues
[Edited on 30-6-2015 by Sulaiman]
|
|
|
macckone
International Hazard
    
Posts: 2159
Registered: 1-3-2013
Location: Over a mile high
Member Is Offline
Mood: Electrical
|
|
U238 is seldom pure and contains decay products in small quantities.
My assumption is that you left those behind in the flask.
You can have beta emitters as well as gamma emitters in small
Quantities. Some elements in the decay by more than one pathway.
Neutron emitters are pretty rare.
http://www.ccnr.org/decay_U238.html
|
|
|
quantumcorespacealchemyst
Banned Shitposter
Posts: 213
Registered: 17-10-2014
Member Is Offline
Mood: No Mood
|
|
macckone, it was unseperated. the 238 chunk that was dissolved is left with the iodine in the flask. there was no seperations. any daughter products
bound in the metal chunk should have entered the liquid phase and now the solid phase; however, the radioactivity seems to only have shown after the
distillation to near dryness.
Sulaiman, yeah, that is interesting. i am still even more of a novice to electronics, however, help exists, at diyphysics.org about how to build a
gammaspectrophotometer PMT detector, there is a hack called the Gamma Grapher i saw from there involving a cheap oscilloscope hack. the unworked out
bits are the power supply for the pmt and building a dynode voltage multiplier.
www.diyphysics.com/2013/04/08/using-surplus-philips-xp2422sn...
http://www.diyphysics.com/2013/03/22/diy-scintillation-probe...
http://www.diyphysics.com/2012/09/21/using-the-79-sainsmart-...
www.diyphysics.com/2012/02/01/open-source-handheld-gamma-spe...
i had trouble with getting electronic money besides paypal so couldn't get from sphere research so went to ebay and even though i saw the detector
listed, i thought the smeared stuff on the PMT face meant it was in unknown condition and went for a better detector (it seems according to the spec
sheets). it turns out, i believe that was only the optical coupling grease (had not seen a PMT in real life before  ). by reading the comparable ranges or sensitivities, it is most certain i got a
slightly better PMT; however ). by reading the comparable ranges or sensitivities, it is most certain i got a
slightly better PMT; however  , i have no idea how to make the dynode voltage
multiplier or make a power supply and keep it from running volatage back to the Gamma Grapher. , i have no idea how to make the dynode voltage
multiplier or make a power supply and keep it from running volatage back to the Gamma Grapher.
if there is a good place to go for this kind of specific learning please let me know. i hope to be able to diagnose the dynode voltage multiplier
improvisation by PMT data sheet information and how to make tunable, stable high voltage power supllies in DC (and also in AC with tunable frequency
although probably not needed for these purposes).
thanks
[Edited on 30-6-2015 by quantumcorespacealchemyst]
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
Often Uranium that is freely sold is U235...
"HOWEVER, after distilling the Acetone out (found it distilled around 80-90ish C so it did not appear to have iodinated the AcMe as i wondered about
and or catalyzed and/or reacted it as i wondered about)"
The boiling point of aceton is 56°C and iodoaceton at 163°C... so at 80-90°C you may have a mix I2, iodoaceton and aceton
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
quantumcorespacealchemyst
Banned Shitposter
Posts: 213
Registered: 17-10-2014
Member Is Offline
Mood: No Mood
|
|
Scientific Illuminism or typo?
PHILOU Zrealone
"Often Uranium that is freely sold is U235..." i don't believe so. i may be wrong
https://en.wikipedia.org/wiki/Natural_uranium
"Natural uranium (NU, Unat[1]) refers to uranium with the same isotopic ratio as found in nature. It contains 0.7% uranium-235, 99.3% uranium-238,
and a trace of uranium-234 by weight (0.0055%). In terms of the amount of radioactivity, approximately 2.2% comes from uranium-235, 48.6% uranium-238,
and 49.2% uranium-234."
the stuff sold is supposedly 238, that is what it said on the packing and the item description. i have not heard of 235 being sold and if it is it
probably clandestine, again, i don't know. i hope to find 115 instead in that case because space travel by rockets is really depressing when the
seeming limits are considered.
"The boiling point of aceton is 56°C and iodoaceton at 163°C... so at 80-90°C you may have a mix I2, iodoaceton and aceton " yea somethings not
right there
Sulaiman
i forgot to mention, that even if it is I125, it wasn't emmiting when in solution. Ithink i even tested it with the detector right before
distillation. and only after going outside and removing the septum and putting the detector above the mouth did it even raise a little and not enough
to even set off the .30microSeivert/hour threshold beep. after sitting for some time in my drawer after distillation, cooling capping and cleaning, i
tested it with the detector and was surprised and exited.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Prove it!
U-235 is the fissionable U isotope, fuelling both civilian and military use of nuclear fission. Its natural abundance (in natural U) is only 0.72 w %
and separation of U-235 (so-called 'enrichment') is extremely difficult. Pure U-235 must be one of the most tightly controlled materials on earth.
The only possibility for non-state actors to possess some U-235 is in the form of natural uranium.
You're blowing through your *rsehole again but what's new?
[Edited on 30-6-2015 by blogfast25]
|
|
|
PHILOU Zrealone
International Hazard
    
Posts: 2893
Registered: 20-5-2002
Location: Brussel
Member Is Offline
Mood: Bis-diazo-dinitro-hydroquinonic
|
|
My mistake!
I thought the U235 (holding less neutrons) was the non-military Uranium thus the remaining from the enrichment.
I have a jar of uranyl nitrate at home and a friend working at the Atomic Energy Circle (CEA) told me that more than certainly it was the non-military
Uranium that must be found in that salt otherwise I wouldn't have been able to buy it!
[Edited on 1-7-2015 by PHILOU Zrealone]
PH Z (PHILOU Zrealone)
"Physic is all what never works; Chemistry is all what stinks and explodes!"-"Life that deadly disease, sexually transmitted."(W.Allen)
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Try fact checking before you hit the keyboard: there's enough bloody crap on Teh Tinkerwebs as it is w/o you adding to it.
|
|
|
Sulaiman
International Hazard
    
Posts: 3555
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
I was not suggesting a new/complex test setup;
since your geiger counter is detecting something
all you need to do is fix the relative positions of the source and counter
and periodically note the reading of the geiger counter.....simple
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Sulaiman  | I was not suggesting a new/complex test setup;
since your geiger counter is detecting something
all you need to do is fix the relative positions of the source and counter
and periodically note the reading of the geiger counter.....simple
|
Not really, actually. The Radex type counter he uses measures combined radiation and isn't energy (that is isotope) specific. For half-life
measurements you need to measure radiation intensity for an isotope-specific type of radiation over time. And for long half-lives that can
take a while too, you know?
Half-life measurements aren't easy at all and a 'household' type meter won't cut it in most cases. It would only really work for a single
radio-isotope and one with no radioactive decay products (so-called 'daughter radio-isotopes').
Other methods involve chemical/physical measurements of (quantities of) radio-isotopes and/or their daughter products, rather than radiation rates.
[Edited on 1-7-2015 by blogfast25]
|
|
|
Sulaiman
International Hazard
    
Posts: 3555
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
If you want absolute results, I agree.
In this case there was a low activity at first, followed by high activity after the reaction,
IF new isotopes were created with measurable half-life
then I assume that within one half-life of whatever was created
the geiger counter reading will reduce.
Identifying what (if anything) was created would need far more sophisticated measurements
the procedure that I suggest is just to determine if there was an isotope of short half-life (e.g. 15.7 days) created.
I'm sure that there will be wide error margins
(replacing geiger in same position, battery condition etc.)
but if the readings are substantially constant for say 30 days then at least some isotopes can be eliminated.
My own suspicion is that the uranium has been spread over the wall of the flask, less water to travel through,
or just a large radiating surface facing the geiger counter etc.
i.e. just a physical distribution effect, not isotope generation
... interesting to see if any results.
or, with three iodine atoms surrounding each uranium atom,
maybe an isotope was created in detectable amounts,
if so then I would expect the geiger counter reading to slowly increase,
a sudden increase seems less likely to me (but my nuclear knowledge is only superficial)
[Edited on 1-7-2015 by Sulaiman]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Sulaiman  | If you want absolute results, I agree.
In this case there was a low activity at first, followed by high activity after the reaction,
IF new isotopes were created with measurable half-life
then I assume that within one half-life of whatever was created
the geiger counter reading will reduce.
Identifying what (if anything) was created would need far more sophisticated measurements
the procedure that I suggest is just to determine if there was an isotope of short half-life (e.g. 15.7 days) created.
I'm sure that there will be wide error margins
(replacing geiger in same position, battery condition etc.)
but if the readings are substantially constant for say 30 days then at least some isotopes can be eliminated.
|
Firstly, the idea that activity was somehow created here, is simply stupid nonsense. There is in that mix nothing that could lead to
nuclear reactions (as opposed to chemical ones, I hope you appreciate the difference) and thus newly created isotopes and newly created activity. In
any case, he would have to do so, so much better to make that case convincingly.
Nuclear reactions that involve absorption of alpha particles are in any case very rare, due to the nature of He<sup>4</sup> nuclei.
| Quote: | IF new isotopes were created with measurable half-life
then I assume that within one half-life of whatever was created
the geiger counter reading will reduce. |
Nope. Not at all. If there was a significant isotope present from the start, with a long half-life (like U-238) then overall activity might only
decrease very slowly and not in accordance the faster decay of the secondary one (especially if the concentrations of the secondary one are small,
always a reasonable assumption in these situations).
I can prove prove all this mathematically, BTW. In fact, I think I will, tomorrow. Stay tuned, if you like.
Quantumcorewhatever is simply riddled with confirmation bias: he WANTS to see something that isn't there. In a peer reviewed environment he'd be
laughed out of court and rightly so. Or shot down in flames, if you prefer that expression.
[Edited on 1-7-2015 by blogfast25]
|
|
|
Sulaiman
International Hazard
    
Posts: 3555
Registered: 8-2-2015
Location: 3rd rock from the sun
Member Is Offline
|
|
I agree with you.
nevertheless I guess that there is the theoretical possibility of iodine isotope creation,
is it worth the effort to disprove it?
(stranger phenomena have been discovered by accident)
just to clear my thinking,
are there no fission products from uranium that could create an iodine isotope from naturally occuring iodine?
[Edited on 1-7-2015 by Sulaiman]
[Edited on 1-7-2015 by Sulaiman]
[Edited on 1-7-2015 by Sulaiman]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by Sulaiman  |
just to clear my thinking,
are there no fission products from uranium that could create an iodine isotope from naturally occuring iodine?
|
Iodine (several isotopes, I believe) is a possible fission product of U-235. But U-238 isn't fissionable, not to mention that there
isn't anything to "provoke" fission, in QC's mix. U-235 needs a neutron (and of a quite narrow energy spectrum too!) to absorb the neutron,
then the 'excited' U-236 nucleus splits into more or less two equal halves (of which iodine isotopes are definitely a possibility).
In Britain the local population is issued with iodine tablets when a new nuclear submarine's reactor goes 'hot' for the first time. Natural iodine
would replace I radioisotopes in a worst case scenario of dissipation and absorption by human metabolism.
https://en.wikipedia.org/wiki/Iodine-131
Fission of U-235 also produces 2 to 3 neutrons (per fission). These can be absorbed by many isotopes (including some I isotopes), resulting in a
radioactive I isotope. But there's no U-235 and no neutrons (to cause U-235 fission) in QC's setup.
[Edited on 1-7-2015 by blogfast25]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
BTW, unless 'Quantumcoreblahblah' moderates his stance on his 'I created radioactivity', or repeats his experiment but much, much 'tightened up',
reports it correctly and draws acceptable conclusions, I will report this thread to moderators with a proposal to move it (or relevant parts of it) to
'detritus' or 'whimsy', where it can't be indexed by Google.
Extraordinary claims require extraordinary evidence and SM should not be the disseminator of pseudo-scientific gobbledegook,
for the vanity of one of its junior members.
[Edited on 1-7-2015 by blogfast25]
|
|
|
quantumcorespacealchemyst
Banned Shitposter
Posts: 213
Registered: 17-10-2014
Member Is Offline
Mood: No Mood
|
|
i have no stance, i have some observations. the title says puzzled because it is a mystery to me.
when i can manage to repeat the reaction with another dedicated flask (the curent one is my storage container for now) or transfer it to a storage
container and repeat in the current cleaned flask, i hope to document it. being that it was an unexpected occurrence i did not document it. my goal
was pure UI3 and to finally/beginning work with Uranium. also i only have 2 similar pieces left and my funds are questionable. i will probably be
doing the experiment again. how soon is a question of a few things one being if i wan't to put my funds into building a gammaspectrophotometer or
rerunning the experiment with a semi blind detector. after all, only putting U metal in another flask may show radioactivity or none and can be used
as a temporary gauge without using metal i have little of hastily. if chemists here are interested in replicating/testing and seeing for themselves
they may be able to get pure 238 from United Nuclear or ebay or elsewhere (United Nuclear only ships Uranium within the United States).
how vain can this be, it's anonymous (i hope)?
a cool idea i am thinking about for the heck of writing it is a custom flask made of a scintillating material that is coupled to a
gammaspectrophotometer while the Uranium is added, then the iodine and then the acetone (or iodine dissolved in acetone is added).
then it is refluxed, with there being a vacuum between the scintillator flask and the PMT so no heating (minimal) occurs (also PMT is cooled). then
solvent (unknowns) are removed by gentle heating under vacuum. all this recorded in real time and analyzed.
anyone with the access to this equipment and/or the ability to hack it together, i hope does and hopefully can even make it known/easier for us/me to
do the same and see it myself and draw my own conclusions or rather hypothesis.
like i said, i don't have a stand besides keep wondering/searching about the truth.
[Edited on 1-7-2015 by quantumcorespacealchemyst]
Sulaiman
"or, with three iodine atoms surrounding each uranium atom,
maybe an isotope was created in detectable amounts,
if so then I would expect the geiger counter reading to slowly increase,
a sudden increase seems less likely to me (but my nuclear knowledge is only superficial)"
that may explain why it did not show activity when dissolved, not being bonded untill evaporation. please let me know, what is the nature of a
dissolved iodide in a solvent that is not water> does it ionize like salts in water or is the presumed UI3 only dispersed while still bonded?
thanks
[Edited on 1-7-2015 by quantumcorespacealchemyst]
[Edited on 1-7-2015 by quantumcorespacealchemyst]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by quantumcorespacealchemyst  |
that may explain why it did not show activity when dissolved, not being bonded untill evaporation. please let me know, what is the nature of a
dissolved iodide in a solvent that is not water> does it ionize like salts in water or is the presumed UI3 only dispersed while still bonded?
thanks
|
This is precisely what you don't seem to grasp: in order for the natural iodine to develop radioactivity some of the nuclei of one or more iodine
isotopes has to be altered. A chemical reaction cannot achieve that in any way, shape or form. This is A level physics, nothing more. Physics 101.
Nuclear reactions take place in the nucleus, chemical reactions take place in the outer electrons of the electron clouds surrounding the nucleus.
Your uranium only contains U-338 and its only mode of decay is α (He<sup>4</sup> nuclei). Very fast neutrons can also fission U-238 but
these are obviously not present here.
He<sup>4</sup> nuclei are doubly charged ions: He<sup>2+</sup>. That double charge and the 53 positive charges of the iodine
nuclei makes for extremely fierce electrostatic repulsion when an α particle approaches an iodine nucleus, making penetration or capture impossible.
Strongly accelerated (using particle accelerators) α particles can be fired into iodine nuclei but again that is not the case here.
As a result, whether the U and I are present as a physical mixture or as a chemical compound (UI3 or UI4) matters not one iota here.
See also: Rutherford's famous alpha scattering experiment. The alphas simply bounce off the gold nuclei.
[Edited on 1-7-2015 by blogfast25]
|
|
|
quantumcorespacealchemyst
Banned Shitposter
Posts: 213
Registered: 17-10-2014
Member Is Offline
Mood: No Mood
|
|
i understand that. i am assuming it is something to do with more surface area being why radiation is detected now compared to when it was in lump
form. i entertain the thought of what if [an unknown] for the reason that when dissolved in solution and containing roughly the same surface area on
the glass, presented to the detector, there was no radiation that i can recall. given is that i don't have a picture of it yada yada; however, it is
weird enough to me to think past the accepted limit of mechanisms or whatever. suppose Sulaiman's suggestion is correct, that the close proximities of
iodine atoms to their core component uraniums are getting blasted easier/effectively somehow. ruthorford used supposed near atom thick gold foil and
the ricochets could rebound without hitting anything besides air or pass through mere atoms of gold. with an emitter in a mass of other emmiters and
equally (somewhat/sortof due to crystal structure and or unreacted iodine possibilities) surrounded satellites, perhaps there is transmutation
occuring. i don't know, has me interested though.
[Edited on 3-7-2015 by quantumcorespacealchemyst]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
What radiation?
https://en.wikipedia.org/wiki/Radiation
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Once an α particle leaves a U-238 nucleus it has a fixed energy (of 4.27 MeV, to be precise). As the Laws of Motion tell us, an object travelling at
constant speed will not lose speed or change direction unless a force make it do that. As a result its kinetic energy of 4.27 MeV is constant and it
doesn’t matter whether an I nucleus in its path is a femtometer or a light year away.
Higher concentration of iodine increases the probability of collisions of course but 4.27 MeV is far too low to overcome the electrostatic repulsion
forces between the 2 protons in the α and the 53 protons in the I nucleus.
Alphas can react with lighter nuclei like N-14 and Al-27 but it takes real bombardment to get some activity.
http://www.3rd1000.com/history/nuclear2.htm
An SMer here tried something like that with Al foil and got disappointed.
[Edited on 3-7-2015 by blogfast25]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
You know how radiation can cause scintillas on an ZnS screen, right? Well, those stars you see with your eyes closed every night have nothing to do
with that: they're CHEMICALLY induced! 
|
|
|
mayko
International Hazard
    
Posts: 1218
Registered: 17-1-2013
Location: Carrboro, NC
Member Is Offline
Mood: anomalous (Euclid class)
|
|
Quote: Originally posted by blogfast25  |
You know how radiation can cause scintillas on an ZnS screen, right? Well, those stars you see with your eyes closed every night have nothing to do
with that: they're CHEMICALLY induced!  |
Usually, though astronauts provide an interesting exception!
| Quote: |
It has long been known that many people in space experience sudden phosphenes, or light flashes. Although it is clear that they are related to
high-energy particles in the space radiation environ- ment, many details about them are still unknown. In an effort to gain more knowledge about the
light flashes, a study was initiated to collect information from people who have recently flown in space. Method: A survey conducted by anonymous
questionnaire was performed among astronauts regarding their experience of sudden light flashes in space. In all, 98 surveys were distributed to
current NASA and ESA astronauts. Results: Among the 59 respondents, 47 noticed them sometime during spaceflight. Most often they were noted before
sleep, and several people even thought the light flashes disturbed their sleep. The light flashes predominantly appear white, have elongated shapes,
and most interest- ingly, often come with a sense of motion. The motion is described as sideways, diagonal, or in-out, but never in the vertical
direction. Dis- cussion: Comparisons with earlier studies of light flashes in space and several ground-based studies during the 1970s are made. One
interest- ing observation from this is that it seems that a small fraction of the light flashes is caused by Cherenkov radiation, while the majority
is probably caused by some kind of direct interaction with elements in the retina. |
Fuglesang, C., Narici, L., & Picozza, P. (2006). Phosphenes in Low Earth Orbit : Survey Responses from 59 Astronauts. Aviation, Space, and
Environmental Medicine, 77(4), 449–452.
[Edited on 4-7-2015 by mayko]
al-khemie is not a terrorist organization
"Chemicals, chemicals... I need chemicals!" - George Hayduke
"Wubbalubba dub-dub!" - Rick Sanchez
|
|
|
| Pages:
1
2 |