loststar0
Harmless

Posts: 8
Registered: 7-7-2015
Member Is Offline
Mood: No Mood
|
|
Pregnenolone sulfate synthesis
Can anyone synthesize pregnenolone sulfate from pregnenolone or get it for under $30 / 150 mg.
[Edited on 7-7-2015 by loststar0]
|
|
|
Praxichys
International Hazard
    
Posts: 1063
Registered: 31-7-2013
Location: Detroit, Michigan, USA
Member Is Offline
Mood: Coprecipitated
|
|
Seems pretty straightforward.
Methyl alcohol is converted to dimethyl sulfate using cold concentrated sulfuric acid. Dimethyl sulfate undergoes partial hydrolosis to methyl
bisulfate.
2x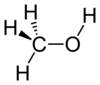 + H2SO4 --> + H2SO4 --> + H2O --> + H2O -->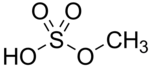 x2 x2
Similarly (hypothetically) with pregnenolone to pregnenolone (bi)sulfate:
 -- same process --> -- same process --> 
I think the main competing reaction may be acid addition of water across that unsaturated bond. The product, being fairly acidic, could be isolated
with a quick a/b extraction. The differences in pKa between the reactant and product probably make it a good candidate for column separation, if TLC
shows it is necessary. Perhaps adsorbtion/desorbtion on a cation exchange column as the sodium salt?
Might be worth a try if the offer is $200/gram. Although Sigma sells the sodium salt for $600/gram...
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
Why is it that nearly every organic chemistry reaction uses sulfuric acid as a catalyst (and requires reflux for days)? What's so special about it?
Why not other acids? Why are acids required at all? Forgive me for my ignorance, but I never took organic chemistry in school nor do I practice it at
the hobby level.
Awaiting the chorus of angry exceptions to my limited (and biased) observations. If you didn't get the hint, I prefer inorganic 
|
|
|
SimpleChemist-238
Hazard to Others
  
Posts: 147
Registered: 28-9-2014
Member Is Offline
Mood: Chlorine Trifloride Flame Thrower
|
|
Sulfuric acid is quite useful, its used as a acid catalyst in a voluminous amount of reactions because of its properties. Its a strong acid and also
can remove water from many reactions. For example in the synthesis of methyl benzoate were water can drive the reaction back yielding the reactants.
For example nitric acid is not used because it often acts like a base, this is why sulfuric acid reacts with it to form the nitronium ion responsible
for nitration.
We are chemists , we bring light to the darkness. Knowledge to ignorant, excitement to the depressed and unknowing. we bring crops to broken fields
and water to the desert. Where there is fear we bring curiosity.
|
|
|
gdflp
Super Moderator
      
Posts: 1320
Registered: 14-2-2014
Location: NY, USA
Member Is Offline
Mood: Staring at code
|
|
Quote: Originally posted by SimpleChemist-238  | | Sulfuric acid is quite useful, its used as a acid catalyst in a voluminous amount of reactions because of its properties. Its a strong acid and also
can remove water from many reactions. For example in the synthesis of methyl benzoate were water can drive the reaction back yielding the reactants.
For example nitric acid is not used because it often acts like a base, this is why sulfuric acid reacts with it to form the nitronium ion responsible
for nitration. |
Nitric acid very rarely acts as a base, the nitration reaction is fairly unique in this aspect. This is not the reason why nitric acid is nearly
never used as an acid catalyst in organic chemistry, it's due to it's highly oxidizing properties, especially at high temperature. Sulfuric acid is
also used because of it's low volatility, as well as it's strength, allowing it to protonate, not necessarily fully but enough for a reaction to
proceed, many common organic compounds such as alcohols, ketones, aldehydes, and esters.
Many organic reactions involve refluxing for several reasons. For one, it allows the temperature to be precisely controlled, determined by the choice
of solvent. The other main reason is that while many simple inorganic reactions are driven by the concentration of ions(simple metathesis reactions
are nearly instantaneous), organic reactions can have a very low reaction rate, increasing the temperature simply allows the reaction to proceed at an
appreciable rate and overcomes the significant activation energy required by most organic reactions.
|
|
|
smaerd
International Hazard
    
Posts: 1262
Registered: 23-1-2010
Member Is Offline
Mood: hmm...
|
|
Interesting Praxichys
I was thinking along the lines of chlorosulfonic acid and a mild base. I guess the enolate could be a problem.
So maybe protect the ketone as a ketal, then chlorosulfonic acid + base, weak acid hydrolysis.
|
|
|
Praxichys
International Hazard
    
Posts: 1063
Registered: 31-7-2013
Location: Detroit, Michigan, USA
Member Is Offline
Mood: Coprecipitated
|
|
It's terribly convenient.
It's a high-boiling, diprotic, strong, slightly-oxidizing, dehydrating acid that forms both soluble and insoluble salts with alkali and alkaline earth
metals.
Because of its high boiling point, it can be used to isolate a huge number of organic and inorganic acids from their salts through distillation. It
dehydrates many organic compounds, forming acid anhydrides and ethers. It can add hydroxyls to alkenes and replace halides with unsaturated bonds. It
can strip hydroxyls to alkenes at high temperatures or form sulfate esters at low temperatures, making great alkylating agents in the process. As a
strong desiccant it has many uses in acid-catalyzed chemistry like esterification. Where lesser acids would boil away during reflux, a Fischer
esterification goes smoothly with H2SO4. Various organic compounds may be forcibly dehydrated to useful products, like formic acid to make carbon
monoxide. As a strong acid, it forces equillibria toward sulfate salts, freeing many organic acids with lesser pKa for liquid/liquid extraction, or
for recharging ion exchange columns. Its high density makes it suitable for drying many organic phases. Sulfonated aromatic compounds gain solubility
and resistance to oxidation, like in a classic picric acid synthesis. Then, when no longer necessary, sulfate can be precipitated as its calcium or
barium salt, leaving an inert product ready for disposal and a nice, clean solution containing your target. Many amine sulfates are solids and easy to
handle - conversely, many amine hydrochlorides are volatile liquids. The acid can be concentrated simply by heating it, allowing its easy reuse.
Sulfonic acids are ubiquitous in azo dyes, which provide color to most of the things one comes in contact with on a daily basis. Most plastics involve
sulfuric acid somewhere in the process of making them, especially nylon and rayon. Long chain organic sulfonic acids are great surfacants in
widespread use, all mode from sulfuric acid. It also has countless applications in batteries, fertilizer, explosives, pesticides and herbicides,
electroplating, and metal refining and surface treatment.
Yes, sulfuric acid... I'd bathe in the stuff if I could. I can easily name it as the single most useful (and probably most used) reagent I have, apart
from water.

|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
Great answers! I appreciate the lesson.
|
|
|