| Pages:
1
..
8
9
10
11
12
..
33 |
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
What do you mean by 'my notes'?
I've been trying to 'transition' my understanding of QM in R<sup>3</sup> to the Hilbert space and am finding it hard. Will try and read
these notes. Ta.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
What's R<sup>3</sup> ?
Edit:
Apologies. Looked it up and found it means Euclidean Space.
Hilbert Space looks pretty much like the bunny, almost.
[Edited on 5-9-2015 by aga]
|
|
|
annaandherdad
Hazard to Others
  
Posts: 387
Registered: 17-9-2011
Member Is Offline
Mood: No Mood
|
|
I wrote them, for a class I used to teach.
Any other SF Bay chemists?
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Wow, so we have a real QMechanic in our midst, perhaps even two with Darkstar. I'm impressed.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Part II: Applications of Wave Mechanics in Chemical Bond Theory
This section will possibly be even more ‘math-lite’ than Part I and will treat chemical bonds mainly from a VSEPR (Valence Shell Electron Pair
Repulsion) point of view.
The Mother of all Covalent Bonds: the σ<sub>ss</sub> bond in Molecular Dihydrogen
Imagine two hydrogen atoms in the ground state (1s<sup>1</sup> approaching each other as in the diagram below: approaching each other as in the diagram below:
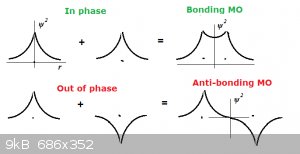
Remember that both wave functions + ψ and – ψ satisfy the Schrödinger equation. In the top part of the diagram the wave functions have the same
sign (either +,+ or -,-) and are said to be ‘in phase’. In the bottom part the wave functions have the opposite sign (either +,- or -,+) and are
said to be ‘out of phase’.
Like actual waves, the wave functions can now show reinforcing interference when they are in phase (top) or negative interference when they out of
phase (bottom).
In the top situation a bonding molecular orbital has formed. See how in the top part the value for ψ<sup>2</sup> between
the nuclei (represented as dots) is positive. This means there is considerable probability of finding the electron(s) in that region and this is where
they reduce the electrostatic repulsion between the (both positively charged) nuclei. A σ<sub>ss</sub> (σ for short) has formed and a
H<sub>2</sub> is born.
But in the bottom part for ψ<sup>2</sup> between the nuclei is zero and with no electron presence in that region there is nothing to
prevent the electrostatic repulsion between the nuclei from ripping the ensemble apart. This orbital is called an anti-bonding molecular
orbital, noted as σ<sup>*</sup>.
Graphical representations of bonding an anti-bonding molecular can be found at the following links:
Bonding:
http://winter.group.shef.ac.uk/orbitron/MOs/H2/1s1s-sigma/in...
Anti-bonding:
http://winter.group.shef.ac.uk/orbitron/MOs/H2/1s1s-sigma-st...
Molecular orbitals must also obey Pauli’s Exclusion Principle, so one electron is the σ orbital must be spin up and the other spin down. Also, the
molecular orbital cannot accommodate a third electron.
In box representation we can write:
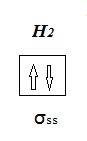
[Edited on 12-9-2015 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Wooohoo ! Applications !
Is sigma ss the sum of the two s orbitals ?
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
In a sense. In textbooks you'll read that 'MOs are linear combinations of AOs'. This 'LCAO' approach is an approximation that works remarkably well.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Bonding and anti-bonding MOs between p-orbitals and between s and p-orbitals
First, a short note on the x, y, z orientation of (the three) p atomic suborbitals. There are three p suborbitals, denoted as
p<sub>x</sub>, p<sub>y</sub> and p<sub>z</sub>:
• when the lobes of the p suborbital lie on the same axis that connects the nuclei of the atoms being bonded, we call that orientation x.
• when the lobes of the p suborbital are perpendicular to the axis that connects the nuclei of the atoms being bonded, we call that orientation y
or z.
Whether or not atomic orbitals can combine into molecular orbital depends (mainly) on two factors:
1. Symmetry considerations which are best illustrated below:
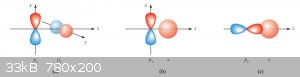
In the cases (a) and (b), lack of symmetry prevents a molecular orbital from being formed. Only in the case (c) is there sufficient symmetry for
bonding to occur.
2. Energy requirements:
For an MO to be formed both contributing AOs need to be of energies not too different (case (c)). MOs resulting from interaction between s an p
orbitals are for that reason comparatively rare (an example is HF, hydrogen fluoride).
σ bonding and anti-bonding MOs formed from two p<sub>x</sub> AOs:
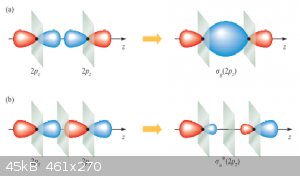
π bonding and anti-bonding MOs formed from two p<sub>y</sub> (or p<sub>z</sub>, that makes no difference here)
AOs:
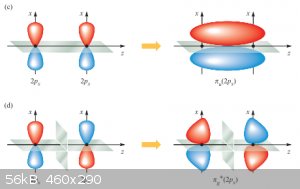
In both figures same-coloured lobes are in phase, different coloured ones are out of phase.
Summarising (as well as over-simplifying) so far we have:
A bonding Molecular Orbital (MO) contains 2 electrons (one spin 'up', one spin 'down'). One is 'donated' by the first atom in the bond, the second by
the second atom in the bond. Bonding MOs only form between electrons that are part of the valence electrons, i.e. the outermost electrons. Inner
electrons are too tightly bound to the nucleus to be able to take part in bonding MOs.
Bonding MOs arise between:
• 2 half filled s atomic orbitals.
• a half filled s atomic orbital and a half filled p<sub>x</sub> atomic orbital.
• 2 half filled p<sub>x</sub> atomic orbitals.
• 2 half filled p<sub>y</sub> or p<sub>z</sub> atomic orbitals. </sub>
These bonding MOs are respectively named:
• sigma (σ) s-s bond. σ<sub>2s</sub> for short.
• sigma (σ) s-p bond. σ<sub>sp</sub> for short.
• sigma (σ) p-p bond. σ<sub>2p</sub> for short.
• Pi (π) p-p bond. π for short.
Here are some ‘artist’s impressions of:
σ<sub>2p</sub>:
http://winter.group.shef.ac.uk/orbitron/MOs/N2/2pz2pz-sigma/...
π:
http://winter.group.shef.ac.uk/orbitron/MOs/N2/2px2px-pi/ind...
[Edited on 14-9-2015 by blogfast25]
[Edited on 15-9-2015 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Cor blogers. You'r into mindf**k territory now.
Assuming that AO means atomic orbitals and MO means Molecular Orbitals.
Do Kinetics comes into play seeing as the Symmetry rule means that no bond can form by kinky approaching atoms, and only head-on approaches form bonds
(vast generalisation). ?
I mean, is the Bond type also influenced by the angle of approach, or is that a function of the structure of the reacting species ?
The naming and the ordering of the bondings seems remarkably clear.
Remarkable in that it seems simple.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
aga:
For now try and forget about kinetics and 'approach angles': right now we're only using QM/WM to predict which molecular orbitals (and thus
also which overall molecular shapes) are possible, not how they might come about in chemical reactions. That's a related yet also different
topic.
Crawling, standing up, walking... maybe running much later! 
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Oh. So it's more about predicting what can react with what, and if/where they could form bonds.
Is this anything to do with the meta / ortho / para stuff in OC (which i believe describes where on a ring bonds are formed) ?
Slitherin' before crawling !
[Edited on 15-9-2015 by aga]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  | 1. Oh. So it's more about predicting what can react with what, and if/where they could form bonds.
2. Is this anything to do with the meta / ortho / para stuff in OC (which i believe describes where on a ring bonds are formed) ?
3. Slitherin' before crawling !
|
1. At this point the only thing we are predicting is what kind of molecular orbital structures are 'allowed' (by QM/WM rules/laws).
Of course that's related to chemical reactions but so far I've only really mentioned one chemical: dihydrogen! Chemical reactions involve the
breaking and forming of chemical bonds.
Think of what I'm doing now as playing with ball and stick models.
2. Aromatic ring structures coming up very soon now.
3. You're very much part of the human zoo!
[Edited on 15-9-2015 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
I await the next instalment with bated gill O2 absorbtion.
[Edited on 15-9-2015 by aga]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Double bonds and triple bonds
Bonding MOs can be combined, between the same bonded nuclei. The double bond in CH<sub>2</sub> = CH<sub>2</sub> (ethene) is
made of one σ s-p and one π p-p bond.
The triple bond in ethyn (acetylene, C<sub>2</sub>H<sub>2</sub> is made of one σ s-p and two π p-p bonds. is made of one σ s-p and two π p-p bonds.
π bonds in fact never occur on their own. That would never be a stable spatial arrangement, as there are no electrons to shield the nuclei from each
other: it would fly apart.
The double bond (e.g. C = C bond) can thus be represented as below, where the sigma p-p electron cloud (MO) provides the shielding of the nuclei, to
prevent the thing falling apart from electrostatic repulsion between the positively charged nuclei:
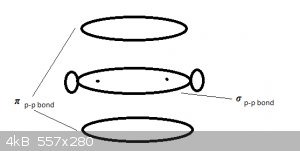
Double and triple bonds also impart torsional rigidity to bonds. Imagine ethane (C<sub>2</sub>H<sub>6</sub> as two bonded methyl groups: H<sub>3</sub>C-CH<sub>3</sub>.
The methyl groups can rotate with respect to each other around the inter-nuclear axis. But if we add a double bond (then we get ethene:
H<sub>2</sub>C=CH<sub>2</sub> as two bonded methyl groups: H<sub>3</sub>C-CH<sub>3</sub>.
The methyl groups can rotate with respect to each other around the inter-nuclear axis. But if we add a double bond (then we get ethene:
H<sub>2</sub>C=CH<sub>2</sub> then the π bond prevents
rotation of the methylene (CH<sub>2</sub> then the π bond prevents
rotation of the methylene (CH<sub>2</sub> groups. Similarly, the CH
groups in ethyn (C<sub>2</sub>H<sub>2</sub>, acetylene) cannot rotate because of the two π bonds. groups. Similarly, the CH
groups in ethyn (C<sub>2</sub>H<sub>2</sub>, acetylene) cannot rotate because of the two π bonds.
Conjugated double bonds and Aromaticity
Lets look at the molecule 1,3 butadiene, CH<sub>2</sub>=CH-CH=CH<sub>2</sub> and number the atoms 1 to 4 from left to right.
The bonds between 1 and 2 and between 3 and 4 are double bonds: a σ<sub>2p</sub> and a π bond. Studies show unequivocally that the two
π bonds do not exist separately but that instead the electron clouds merge:
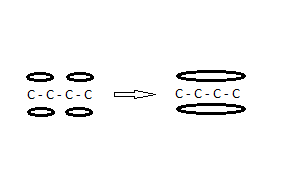
The hyphens represent the unaffected sigma bonds. Both lobes of the merged π bonds together contain a total 4 electrons (2 spin up, 2 spin down). The
bond lengths of 1-2, 2-3 and 3-4 are identical.
Wherever double bonds are conjugated, for example
CH<sub>3</sub>-CH<sub>2</sub>-CH=CH-CH=CH-CH<sub>2</sub>-CH<sub>3</sub> (3,5 octadiene), this merging
occurs. Perhaps the best known example is benzene, C<sub>6</sub>H<sub>6</sub>, where three conjugated bonds merge into a
single π torus electron cloud:
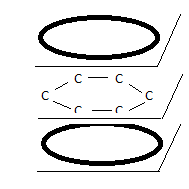
Hydrogen atoms, each bonded with one sigma bond to the C atoms, are not shown here. Both annular electron clouds contain a total of 6 electrons (three
spin up, three spin down). All bond lengths are identical.
However, both in the case of conjugated dienes and benzene , this electron cloud overlapping must not be seen as a linear combination or the
respective π MOs. Such a linear combination would strongly violate Pauli’s rule of only two spin-opposing electrons per MO.
Below an image of the iso-probability density surfaces for the π electrons (as based on a real calculation with ORCA software). The smoothness and
continuity of the orbital surfaces creates the illusion of orbital merger but in reality the three π orbitals continue to exist, each with only two
paired electrons.
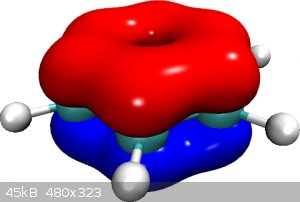
[Edited on 16-9-2015 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
So, if i got this right, the nucelii in a molecule are sandwiched together by electrons whizzing about in the various orbitals that surround them.
No orbital can contain more than 2 electrons : 1 in spin Up the other in spin Down state.
Where those systems are stable defines which and how the molecular nucleii can be contained inside that system.
Not sure why the py and pz bond is called a Pi bond, seeing as it's just a Sigma bond with different orbitals, with the naming being seemingly
arbitrary ...
... then i had another look at https://www.youtube.com/watch?v=K-jNgq16jEY
The dz<sup>2</sup> orbital appears to be the first torroidal-containing orbital, which would kind of cement the whole orientation thing,
fixing where x y and z have to be.
Ok. Hands up.
Still not clear why the py/pz or py/py or pz/pz bond is called a Pi bond instead of a Sigma pyz/pzz/pyy bond.
[Edited on 19-9-2015 by aga]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Aha !
Is it the case that a Sigma bond forms first, then there simply is no geometric room/space left to afford anything other than a Pi bond ?
I.e. the 'mating' px-es are occupied, leaving only the py or pz available for further bonding along that axis, and in that direction.
[Edited on 19-9-2015 by aga]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
aga:
The difference between σ and π MOs is non-trivial and not just a matter of fancy names. It should become even clearer after the next instalment but
for now I’ll say this.
In all cases 1) to 5) below:
• Nuclei approach via the x-axis (x-axis is inter-nuclear axis)
• Atomic Orbitals contain 1 spin up electron (first atom) and 1 spin down (second atom).
• Atomic Orbitals are in phase.
1) Two 1s orbitals approach and interact to form a bonding σ(ss) MO.
2) One 1s orbital and 1 p<sub>x</sub> orbital approach and interact to form a bonding σ(sp) MO.
3) Two p<sub>x</sub> orbitals approach and interact to form a bonding σ(pp) MO.
4) Two p<sub>y</sub> orbitals approach and interact to form a bonding π(pp) MO.
5) Two p<sub>z</sub> orbitals approach and interact to form a bonding π(pp) MO.
(note that p<sub>y</sub> or p<sub>z</sub> orbitals cannot interact with s orbitals, due to poor symmetry (lack of overlap),
see also next instalment)
In short, σ MOs are formed when the interacting atomic orbitals have electron density symmetry around the inter-nuclear
axis. p<sub>y</sub> and p<sub>z</sub> have electron densities that lie above and below the inter-nuclear axis, so
they can’t form σ MOs, only π MOs.
[Edited on 20-9-2015 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
OK. Thanks for the explanation.
Seems pretty clear (best check) :-
s-s, s-px, px-px bonds are Sigma bonds.
py-py, py-pz, pz-pz bonds are Pi bonds.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Amen to that!
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Sorry if this sounds silly : it seems clear that a Pi bond could never be a simple shape like a sigma bond orbital could be.
The maths are beyond me, however the principles (and pictures) seem very clear.
Given that the Py and Pz orbitals are oriented at angles to the Px axis, and are dislocated/unconnected in any case, the toroidal orbital
above-and-below seems like a fair guess at how they'd look.
Personally i imagine it would be a ring, but not uniform in radius or width.
As there is now a second attractant (the second nucleus) surely the opposite side of the orbital would be shortened, creating more of an eliptical
orbital rather than a spherical one.
Hmm. Even with an s-s bond the distance from the 'edge' of the orbital (if there is such a thing) to the nucleus should be reduced when compared to an
unbonded atom.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
aga:
Try not get too hung up on the shapes: those shiny, hard looking surfaces are only surfaces of iso ψ<sup>2</sup> values. Orbitals are
'fuzzy' objects, they have no clear boundaries.
We use these shapes to demonstrate and illustrate how AOs interact with each other to form MOs.
[Edited on 21-9-2015 by blogfast25]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Molecular shapes and molecular orbital repulsion
Let’s look the bond structure and shape of the water molecule. Oxygen’s ground state is 2s<sup>2</sup> 2p<sup>4</sup>.
According to Hund, the four 2p electrons are distributed as 2 paired in p<sub>x</sub> and the two remaining unpaired ones in a
p<sub>y</sub> and a p<sub>z</sub> orbital. These latter ones form σ(sp) with the 1s<sup>1</sup> hydrogen
orbitals, which would strongly suggest a bond angle (between the H-O bonds) of 90 degrees, as in the Lewis structure below (note that Lewis notation
isn’t really intended to show bond real angles):
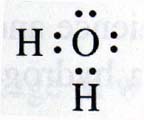
In reality, the structure is closer to that of a tetrahedron, with the two hydrogens in two corners and the two lone paired AOs in the remaining two
corners. That would give a bond angle (between the HO bonds) of 109.5 degrees.
Experiment finds these bond angles to actually be slightly smaller: 104.5 degrees.
The reason that the bond is neither 90 nor 109.5 degrees is due to electrostatic repulsion between the electron pairs (AOs or MOs): as electrons are
negatively charged and each AO/MO contains 2 of them, electrostatic repulsion between them is inevitable.
The repulsion between electron pairs (orbitals) decreases in the following order:
Lone pair-lone pair > lone pair-bonding pair > bonding pair-bonding pair.
The larger repulsion between the two lone pair electrons, for instance in the case of water, causes the HO bond angle to be 104.5 degrees.
Molecular shapes often reflect this: molecular shape tries to minimise inter-MO repulsion because that decreases potential energy of the structure.
Below are the expected molecular shapes for EX<sub>n</sub> -type molecules (X = an exoatom, like H or a halogen):
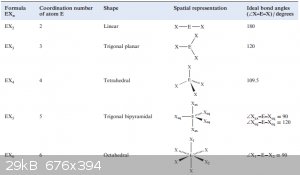

[Edited on 21-9-2015 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Cor bloggers. Your're laying out so flat even i can follow it !
Yous should be Paid well to be a lecturer somewhere.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Nah, I'm rubbish at crowd control.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
No need.
I feel sure you'd be able to make 2-Chlorobenzalmalononitrile for that.
If you're an IOC purist, H2S would do.
|
|
|
| Pages:
1
..
8
9
10
11
12
..
33 |