| Pages:
1
..
12
13
14
15
16
..
33 |
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
| Quote: | | 1 member supplied 2 correct answers so far (20/20). |
Was it you ?
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
No. Now get back to your desk, young man!
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Over 80 % score so far by respondents on the first 2 questions, despite the fact that the answer to Question 2 was far harder to find in the seminar
than I intended. I will now add two graphics that should clarify the matter, so newcomers can earn themselves some really easy points, so what are you
waiting for? Both questions remain open till Sunday coming.
Hydrogen Energy Levels:


Again, apologies for not having included this material earlier on. The Table of Content will also be updated shortly to list this post.
[Edited on 20-10-2015 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
80%
Sorry for dragging the average down guys !
You gotta publish a table of results at the end.
Oooo yeah.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
I will. Respondents can remain anonymous in the table, if they prefer...
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Leave mine as 'aga'.
Let's see how many of these bright sober young sparks can beat a dim drunken old fool.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
This one is to confound everyone, including myself (hehe):
| Quote: | In the end, I think the simplest and more correct single way to categorize the terms is to interpret "particle" as "excitation of a field". For
example, if someone says
"There are two electrons in this box."
I would mentally translate that to
"The electron field in this box has two units of excitation." |
Source.
So particles are 'excitations of the Quantum Field'.
Ding dong.
[Edited on 20-10-2015 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Sounds like a couple of simple agaspace expressions to me.
If you can accept that electrons are not solid balls of matter, nicely making the wave/particle duality easier to accept, groovy.
Then extrapolate briefly.
A new and amazing model appears.
Then disconnect from the Fixed Viewpoint of Linear Time and it all goes ape (in a good way) a.k.a. agaspace.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  |
Then disconnect from the Fixed Viewpoint of Linear Time and it all goes ape (in a good way) a.k.a. agaspace. |
Heavily curved, that agaspace, is it?
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
In some cases.
Grab hold of the odd complex of the X Y Z axes and Time curves to a perfect circle.
Grab onto the Time axis and the X Y Z forms a sphere which gets bigger and then smaller - it oscillates.
Grab onto the Magnetic axis and it's a mind masher.
Grab the Gravitic axis and imagining anything is almost impossible without 12 cans of Finkbrau.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Sorry, but my daughter bought me 'Quantum Mechanics' by F.Mandl (WILEY) for my bufftday and I can't find none of your stuff in there!  She's taking the subject as a 3rd year astrophysics student this year, so I guess
we'll be able to discuss your Tourette's, I mean theory. She's taking the subject as a 3rd year astrophysics student this year, so I guess
we'll be able to discuss your Tourette's, I mean theory.
[Edited on 21-10-2015 by blogfast25]
|
|
|
fluorescence
Hazard to Others
  
Posts: 285
Registered: 11-11-2013
Member Is Offline
Mood: So cold outside
|
|
So blogfast suggested that I should put up the question here.
As some may know when it's about coordination chemistry many theories meet and combine, Valence-Bond, Molecular Orbitals, Crystal Field and so on. But
depending on what you are looking for you will mostly get only an answer from one of these theories and thus it's quite hard to combine all of them in
a single theory or explain certain behaviour without changing between theories too often.
The most common Coordination Numbers are probably 4 and 6 with the Tetrahedron / square planar and the Octahedron. Besides the well known ligand field
splitting we know about stuff like "Hybrids", too.
An Octahedron will form a d²sp³ - Hybrid. If you look that shape up that's totally looking like an octahedron. A tetrahedron should actually form an
sd³ - Hybrid ( some books say sp³ for a tetrahedron but that is for main group elements only). Then there are some like trig. pyramidal (sd²).
Now I was looking for high Coordination numbers and the highest one known that is formed by a monodentate ligand would be a Nona-Coordinated Compound.
[XH9]2- where X = Tc, Re
The compound is perfectely stable forming a tricapped trigonal prism.
Analysis shows that 6 H - Ligands seem to be equal and then there is a second set of 3 H -Ligands. So the question is how that hybride is formed and
whether it's really only related to d-Orbital Coordinations.
If you look at the Structure of that compound it looks a bit like an octahedron that is distortet by 3 other Hydrides.
So is it a d²sp³ and then there are 3 more Ligands that will sort of change the shape or is there a hybride for 9 Ligands ?
I can't really find anything on that topic.
[Edited on 23-10-2015 by fluorescence]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by fluorescence  | So blogfast suggested that I should put up the question here.
As some may know when it's about coordination chemistry many theories meet and combine, Valence-Bond, Molecular Orbitals, Crystal Field and so on. But
depending on what you are looking for you will mostly get only an answer from one of these theories and thus it's quite hard to combine all of them in
a single theory or explain certain behaviour without changing between theories too often.
The most common Coordination Numbers are probably 4 and 6 with the Tetrahedron / square planar and the Octahedron. Besides the well known ligand field
splitting we know about stuff like "Hybrids", too.
An Octahedron will form a d²sp³ - Hybrid. If you look that shape up that's totally looking like an octahedron. A tetrahedron should actually form an
sd³ - Hybrid ( some books say sp³ for a tetrahedron but that is for main group elements only). Then there are some like trig. pyramidal (sd²).
Now I was looking for high Coordination numbers and the highest one known that is formed by a monodentate ligand would be a Nona-Coordinated Compound.
[XH9]2- where X = Tc, Re
The compound is perfectely stable forming a tricapped trigonal prism.
Analysis shows that 6 H - Ligands seem to be equal and then there is a second set of 3 H -Ligands. So the question is how that hybride is formed and
whether it's really only related to d-Orbital Coordinations.
If you look at the Structure of that compound it looks a bit like an octahedron that is distortet by 3 other Hydrides.
So is it a d²sp³ and then there are 3 more Ligands that will sort of change the shape or is there a hybride for 9 Ligands ?
I can't really find anything on that topic.
[Edited on 23-10-2015 by fluorescence] |
Firstly, have a look at the 'ball and stick' model of [ReH<sub>9</sub>]<sup>2-</sup>:
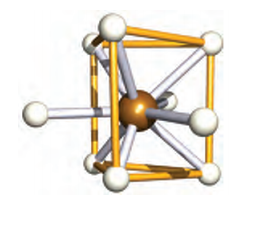
Which is indeed a tricapped trigonal prismatic.
Now have a look at this:

According to it, the structure is explained by an sp<sup>3</sup>d<sup>5</sup> hybridisation, the involved AOs are
shown too. Re in +7 oxidation state.
Source.
As regards,
| Quote: | | But depending on what you are looking for you will mostly get only an answer from one of these theories and thus it's quite hard to combine all of
them in a single theory or explain certain behaviour without changing between theories too often. |
Sure, there are competing theories and they don't all 'marry' seamlessly. That should not worry us though: the point is to have theories that
have predictive power, for now that is all we can hope for.
[Edited on 23-10-2015 by blogfast25]
|
|
|
fluorescence
Hazard to Others
  
Posts: 285
Registered: 11-11-2013
Member Is Offline
Mood: So cold outside
|
|
Thank you for that chart ! I was looking for something like that !
Edit:
Just saw that the chat says sp³, too with the Tetrabromo-Complex.
But shouldn't there actually be d-Orbitals involved in that ?
[Edited on 23-10-2015 by fluorescence]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by fluorescence  |
Just saw that the chart says sp³, too with the Tetrabromo-Complex.
But shouldn't there actually be d-Orbitals involved in that ?
|
Why? Four ligands means four dative bonds, requiring four receptive atomic orbitals: one s + three p = sp<sup>3</sup>.
[Edited on 24-10-2015 by blogfast25]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Last hours of daylight to answer the first two QMQuiz questions!
Answers to these and next two questions tomorrow.
[Edited on 25-10-2015 by blogfast25]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Question 3: (for 30 points)
A particle finds itself in a one dimensional triangular potential well as shown below.
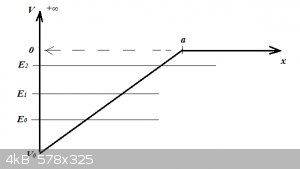
For x = or < 0, V = +∞ ('x equal to or smaller than zero')
For x > a, V = 0
For 0 < x < a, 0 > V > - V<sub>0</sub>
Assume that in the region 0 < x < a, the Schrödinger Equation has three eigenfunctions ψ<sub>0</sub>, ψ<sub>1</sub>
and ψ<sub>2</sub> with corresponding eigenvalues E<sub>0</sub>, E<sub>1</sub> and E<sub>2</sub>.
Sketch what the three wave functions are likely to look like. Briefly explain your reasoning. Sketches do not have to neat, smooth or to scale but
must show zero points and tunnelling where applicable.
Hint: look for parallels with the wave functions for P1DB and Quantum Harmonic Oscillator.
Question 4: (for 10 points)
Below are the structures (hydrogen not shown) of 2,3 dichloro naphthalene:
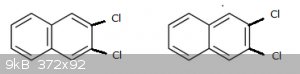
They would suggest two such compounds (two isomers) exist but in reality only one 2,3 dichloro naphthalene is known.
Explain why this is so.
[Edited on 26-10-2015 by blogfast25]
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Electrophilic Addition Reaction Mechanism
On aga’s request I’ll extend the applications of QM/WM in chemistry to a number of explanations of simple reaction mechanisms (mainly drawn from
Organic Chemistry). I’ll start with an Electrophilic Addition Reaction (EAR).
As we’ll see in much greater detail, in an EAR a permanent dipole molecule is added to a double bond.
The permanent dipole molecule X-Y can be represented as below:
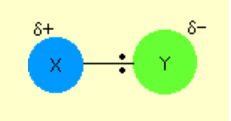
Before we proceed I want to drive home what makes a dipole a dipole a little harder, using QM arguments.
Below is depicted the ground state wave function ψ<sub>1</sub> (idealised, not to scale and reduced to 1 dimension) of a bonding
molecular σ between two X atoms (one on the left, one on the right), so there’s no difference in electronegativity:
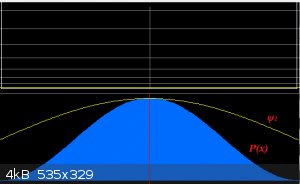
The wave function is perfectly symmetric with respect to the line of symmetry of the molecule. Remember also that the electron density (probability
density distribution) is given by:
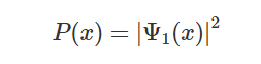
Which in the diagram is represented by the blue ‘bell’. It is perfectly symmetric and the electrons show no preference over the left or right side
of the orbital.
Now consider the case where the left hand side is X, the less electronegative atom, and the right hand side is Y, the more electronegative atom:

It can be argued that the difference in electronegativity introduces a potential V(x) across the orbital, represented by the white sloped
line. This potential affect the potential energy term of the Hamiltonian operator:
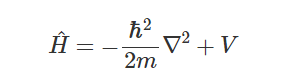
So, the consequence of this potential is that it changes both ψ<sub>1</sub> and P(x): the electron density (green ‘bell’) is now
skewed much more to the right and the X-Y molecule is a permanent dipole.
The mechanism of the EAR is so well explained (at least at the beginner level) in the link below that there nothing to add to it:
http://www.chemguide.co.uk/mechanisms/eladd/whatis.html#top
[Edited on 27-10-2015 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Quote: Originally posted by blogfast25  |
Question 4: (for 10 points)
Below are the structures (hydrogen not shown) of 2,3 dichloro naphthalene:
They would suggest two such compounds (two isomers) exist but in reality only one 2,3 dichloro naphthalene is known.
Explain why this is so.
|
Which one is then 2,3 form please ?
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
They both are. In both cases the two Cl atoms are in positions 2 and 3 (by IUPAC's rules). Don't get hung up about that: this is a game of 'spot the
difference', then explaining why it's not a difference!
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Phew ! Q3 is tricky.
Hardest thing is Drawing the damned thing !
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Sure. It takes a steady hand. It might be bit early for you. 
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
A glimpse into the reaction mechanism of the synthesis of Indole-3-acetic acid
Previously I indicated that the reaction between indole and glycolic acid was a simple EAR (as described above) but it’s not. The orgsynth page:
http://www.orgsyn.org/demo.aspx?prep=CV5P0654
... does not show water as a leaving group but that definitely happens. Also, the double bond is preserved.
The reaction proceeds in three steps:
1) Saponification of the glycolic acid with KOH:
This is a simple acid/base reaction:
HOOC-CH2-OH + KOH === > KOOC-CH2-OH + H2O.
The actual reactant in step two is thus <sup>–</sup>OOC-CH2-OH (the glycolate anion), not glycolic acid itself.
2) The breaking and making of molecular bonds (MOs):
In good old fashioned OC style we’ll try and work out what happens by concentrating only on the reactive sites of the respective reactants.
The glycolate can be re-written as R<sub>1</sub>-CH<sub>2</sub>-OH, the indole as
R<sub>2</sub>-CH=CH-R<sub>3</sub>.
One possible mechanism is symbolised as follows:
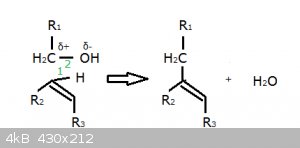
Bond 2 is strongly polarised due to the difference in electronegativity of C and O. The C atom is partially positively charged and is thus an
electrophile.
This carbon atom now ‘snatches’ orbital 1, while the proton in that bond now becomes bonded by orbital 2, thus forming water as the leaving group.
3) Neutralisation:
After cooling the resulting mix is neutralised with HCl solution. Note that R1 = -COO<sup>-</sup>, so we get:
-COO<sup>-</sup> + H<sub>3</sub>O<sup>+</sup> === > -COOH + H<sub>2</sub>O.
With the rest of the structure, that is indole-3-acetic acid which is water-insoluble and precipitates out.
Note that the reaction conditions are fairly harsh, which indicates fairly high activation energy. Tentatively we can explain this by the fact that
R1, R2 and R3 are fairly bulky groups that greatly reduce the number of reactive collisions.
[Edited on 28-10-2015 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Wow !
This is actual Science APPLIED and not just random bullshit.
Could this be the Beginning of SM returning to being an actual Science & Chemistry forum ?
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  | Wow !
This is actual Science APPLIED and not just random bullshit.
Could this be the Beginning of SM returning to being an actual Science & Chemistry forum ? |
No comment.  
For some excellent MO based explanations of OC mechanisms, I can only recommend this page:
http://www.chemguide.co.uk/mechmenu.html#top
I'll be looking for some really amazing applications of QM/WM in chemistry/biochemistry that should really blow anyone's top, so stay tuned!
|
|
|
| Pages:
1
..
12
13
14
15
16
..
33 |