| Pages:
1
..
13
14
15
16
17
..
33 |
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
The Quantum Theory of Hydrogen Bonding
What?!? More stuff about the H-H bond? Haven’t we already covered that? Yes but here we will be talking about something very different but no less
important.
Consider the following structures and their positions respective to each other:

The right hand structure contains an N atom with a lone electron pair (non-bonding orbital) and in principle could bond a proton by donating its
non-bonding orbital (thereby acting as a Lewis base). Of course the left hand structure contains a proton.
If the (left) proton were to cross the gap between the two structures (and become bonded by the right hand lone pair), we could symbolically write
this as:
N:H-----:N === > N:-----H:N, where the dotted line represents the gap between the structures.
Quantum mechanically the situation can be represented by a double potential well coupled by a barrier:
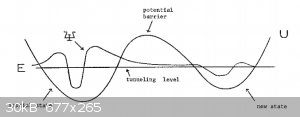
(Note that the wave function ψ here is the wave function of the proton!)
The proton can travel from one well (left N atom) to the other (right N atom) by tunnelling through the barrier (the gap).
But once it’s travelled from NH to N , symmetry dictates that it can also travel back the same way, so we can write:
N:H-----:N <=== > N:-----H:N
The wave function of the proton indicates that it has high probability to the left of the gap, also to the right of the gap but also limited
probability in the gap.
This essentially described hydrogen bonding and it occurs wherever protons and unbounded electron pairs are present. Apart from the N-based example
above, hydrogen bonding contributes to the comparatively high boiling points of water and simple alcohols.
Hydrogen bonding also plays an important part in the structure of ice:
http://www1.lsbu.ac.uk/water/hexagonal_ice.html
It also part explains the structure of DNA, more about that in a following segment.
[Edited on 28-10-2015 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Mind : boggled.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Lewis acid base theory (digest) and an example of OC catalysis explained
| Quote: | A Lewis base is a compound that can donate an unbonded lone electron pair.
A Lewis acid is a compound that can receive an unbonded lone electron pair. |
Typical Lewis bases include NH3, amines, water, hydroxide anions and many other ligands encountered in the formation of coordination complexes. NH3 for example has one lone electron pair to donate, water (potentially) has two.
Perhaps the ‘ultimate’ Lewis acid is the naked proton itself, so keen to receive a lone electron pair that it almost never occurs as a free
species. In water the concentration of free protons is exceedingly small due to:
H<sup>+</sup> + H<sub>2</sub>O === > H<sub>3</sub>O<sup>+</sup>
Other common Lewis acids include many metal cations because when they form coordination complexes they receive lone electron pairs from the Lewis base
ligands.
Of particular interest as Lewis acids are a number of metal halides, MXn, like AlCl3, FeCl3, SnCl4, SbCl5 and SbF5 (list is far from exhaustive). Due
mainly to cost reasons AlCl3 is probably the most popular of that array.
AlCl3 is one of these metal halides that rides the cusp between covalent and ionic compound (just look at its low MP and BP, for instance). It arises
when Al’s sp<sup>2</sup> hybridisation forms sigma bonding MOs with Cl’s lone 3p<sub>z</sub> electrons, forming a planar
triangular structure with minimised inter-orbital repulsions. See diagram below left:
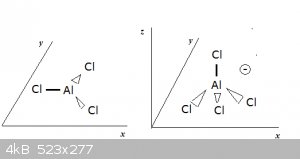
In chloride ions (Cl<sup>-</sup> the Cl nuclei are surrounded by four
full lone pair orbitals (as well as the inner electrons, of course), making the chloride ion a potential Lewis base. the Cl nuclei are surrounded by four
full lone pair orbitals (as well as the inner electrons, of course), making the chloride ion a potential Lewis base.
A chloride ion can donate a lone electron pair to AlCl3, provided the latter undergoes a transition to an sp<sup>3</sup> hybridisation,
leaving one of the sp<sup>3</sup> MOs empty. The chloride ion then donates one of its lone electron pairs into the empty
sp<sup>3</sup> MO and an AlCl<sub>4</sub><sup>-</sup> (tetrachloro aluminate anion) is born. It has a tetrahedral
structure and carries one unitary negative charge (diagram above right). Salts of it, like KAlCl<sub>4</sub>, are known.
This property of AlCl3 (and other suitably Lewis acidic metal halides) is exploited in OC to create highly electrophilic species for use as
‘attackers’ in specific OC substitution reactions.
For example, if we take an alkyl chloride like propyl chloride (1-chloro propane, IUPAC, CH<sub>3</sub>-CH<sub>2</sub>-
CH<sub>2</sub>-Cl ) the chlorine atom is still surrounded by three lone electron pairs and acts as a Lewis base:
CH<sub>3</sub>-CH<sub>2</sub>- CH<sub>2</sub>-Cl + AlCl<sub>3</sub> < === >
CH<sub>3</sub>-CH<sub>2</sub>- CH<sub>2</sub>+ +
AlCl<sub>4</sub><sup>-</sup>.
The last carbon atom now carries a positive unitary charge and we call it a carbocation or carbonium ion. Such a species is extremely
electrophilic.
The case of AlCl<sub>3</sub> catalysed alkylation of benzene is well explained in the link below (but the choice of alkylating agent is a
little odd because methyl chloride is a gas – BP = -24 C):
http://www.chemguide.co.uk/mechanisms/elsub/fcalkyl.html#top
[Edited on 30-10-2015 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
For the dimmest of us, could you please explain what is meant by 'lone electron pair'.
This part of the whole Lewis thing has been confusing for ages.
Is it a Pair that are alone, two seperate Lone lectrons that come together as a Pair that were Lone before meeting etc ?
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  |
For the dimmest of us, could you please explain what is meant by 'lone electron pair'.
This part of the whole Lewis thing has been confusing for ages.
Is it a Pair that are alone, two seperate Lone lectrons that come together as a Pair that were Lone before meeting etc ? |
A lone electron pair is a full molecular orbital (2 electrons) that is not involved in any bonding:
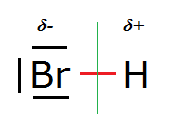
In the diagram the three short lines left and above/below the Br symbol symbolise the lone electron pairs.
Lewis isn't just known for his acid/base theory, he also 'invented' a notation in which electron pairs are symbolised by : (two
dots), for example in this representation of water:
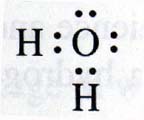
The pairs that aren't located between atoms are lone, unbonded electron pairs.
I refer also to an earlier post about water and the oxonium ion.
In its most basic form a Lewis acid base reaction can be represented as:
B: + A === > B-A, where the hyphen stands for a bonding MO between A and B.
B is the electron pair donor (base) and A is the receptor (acid).
[Edited on 30-10-2015 by blogfast25]
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Oh !
So two electrons, one upspin, one downspin in a single orbital that is Currently not involved in any bonding ?
So a Lewis acid/base could be a molecule that happened to have either a full orbital Not involved in some bondage, or an Empty orbital equally Not
involved (although it wouldn't be, seeing as it would have to be empty) ?
Sorry to labour the point, just that you Know and i don't.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  | Oh !
1) So two electrons, one upspin, one downspin in a single orbital that is Currently not involved in any bonding ?
2) So a Lewis acid/base could be a molecule that happened to have either a full orbital Not involved in some bondage, or an Empty orbital equally Not
involved (although it wouldn't be, seeing as it would have to be empty) ?
|
Yes to 1).
Yes to 2).
Why do we call them Lewis acids and bases? Because there's much overlap with Bronsted-Lowry acid base theory. E.g. NH3 is a base in both theories, so
is water and some others. But the overlap isn't 100 % and Lewis theory is particularly useful in the Quantum explanations of OC reaction mechanisms.
QED with the AlCl3 catalysed alkylations, part of the Friedel-Crafts family of electrophilic substitutions.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Woohoo ! I got something right !
Gotta be worth another point in the scoring 
Yout got to try, no matter what.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
No but I have a much better idea: include a L-AB question in the exam.
Hehehe.
Keep studying. If you think you've understood it, you've probably not understood it enough!
[Edited on 30-10-2015 by blogfast25]
|
|
|
Darkstar
Hazard to Others
  
Posts: 279
Registered: 23-11-2014
Member Is Offline
Mood: Sleepy
|
|
I cannot believe that I just typed all of this... Man, I better get some SERIOUS extra credit or something. This is mostly for you,
aga, so I hope it helps you in some way. I tried to explain the reaction and concepts as best as I could while also keeping it
beginner-friendly, making sure to include lots of details that will help you better understand the amazing world of organic chemistry. If there is
anything at all that you do not understand, please don't hesitate to ask. This post is extremely long, so I don't want you to feel obligated to read
it right away or in one sitting or anything. Just read it when you have the time.
Quote: Originally posted by aga  | Wow !
This is actual Science APPLIED and not just random bullshit.
Could this be the Beginning of SM returning to being an actual Science & Chemistry forum ? |
In light of blogfast's recent posts, I will also post a reaction mechanism and explanation to further help you apply what you're
learning here. If you recall, you made a thread on the synthesis of ethyl acetate earlier this year. Since you weren't able to fully understand the synthesis back then due to your lack of organic
chemistry knowledge at the time, I think now might be a good time to give it another go; however, before we do, we need to make sure that you
understand a few things first:
I know this is a lot to take in, and I'm sure at least some of it you already know, but it's very important that you fully
grasp this stuff before seriously attempting to make sense of the mechanism below (or any OC mechanism, for that matter). I literally intend to
explain, in detail, what's going on in every single step for you, so the more you understand going into my explanation, the more you'll get out of it
in the end.
A. You need to know what skeletal structures are and what they represent. In OC, molecules are drawn using the line-angle formula. This is basically just a short-hand way
to draw carbon chains, as drawing them in this manner is extremely quick and convenient, especially when the chain is long. With the exception of
certain functional groups, carbons and hydrogens are not shown because they are already implicit within the "lines" of the skeletal structure itself.
An exception are hydrogens that are directly bonded to non-carbon atoms. In those cases, it is correct to show the hydrogens explicitly. But other
than that, the only atoms that should be explicit are heteroatoms (atoms other than hydrogen and carbon). Where the lines begin and end are where the
carbons are. The lines connecting those carbons to other atoms represent bonds. The number of lines indicates the number of bonds. By counting the
number of bonds that are connected to a given carbon atom, the number of implicit hydrogens on it can also be determined.
B. You also need to know how many bonds each atom needs in order to become a neutral species.
In this case, we're only dealing with hydrogen, carbon and oxygen:
- Hydrogen needs one bond.
- Carbon needs four bonds.
- Oxygen needs two bonds.
Thus an oxygen that only has one bond (like a hydroxide ion, OH<sup>–</sup> becomes negatively charged because it has one more electron orbiting it than it has protons in its nucleus. Likewise, an oxygen with three
bonds (like a hydronium ion, H<sub>3</sub>O<sup>+</sup> becomes negatively charged because it has one more electron orbiting it than it has protons in its nucleus. Likewise, an oxygen with three
bonds (like a hydronium ion, H<sub>3</sub>O<sup>+</sup> has
a positive charge because it is deficient one electron. Also, keep in mind that a single bond counts as one bond, a double bond as two, and a triple
bond as three. Thus an atom that needs four bonds could either have four individual single bonds, two double bonds, one double bond and two single
bonds, or one triple bond and one single bond. (quadruple bonds technically exist as well, but they're not exactly common) So assuming that it's a
neutral species, a carbon that is bonded to another carbon through a single bond as well as an oxygen through a double bond must then have one
implicit hydrogen. Such a group is known as an aldehyde group. (just as an FYI, even though it's connected to a carbon atom, it's actually correct to show the hydrogen in an aldehyde group
explicitly if you want to. personally, I always show it because aldehydes look goofy without it for some reason) has
a positive charge because it is deficient one electron. Also, keep in mind that a single bond counts as one bond, a double bond as two, and a triple
bond as three. Thus an atom that needs four bonds could either have four individual single bonds, two double bonds, one double bond and two single
bonds, or one triple bond and one single bond. (quadruple bonds technically exist as well, but they're not exactly common) So assuming that it's a
neutral species, a carbon that is bonded to another carbon through a single bond as well as an oxygen through a double bond must then have one
implicit hydrogen. Such a group is known as an aldehyde group. (just as an FYI, even though it's connected to a carbon atom, it's actually correct to show the hydrogen in an aldehyde group
explicitly if you want to. personally, I always show it because aldehydes look goofy without it for some reason)
C. While I didn't bother to actually show the lone pairs on the atoms in the mechanism below,
keep in mind that, like the implicit carbons and hydrogens, the lone pairs are there even though you can't see them. Remember that oxygen can form up
to three bonds to give a positively-charged oxonium ion, so a neutral oxygen with two bonds still has another lone pair that can participate in
bonding. Understanding this is going to be a central part of understanding the mechanism below.
D. Lastly, as I've mentioned before, the red arrows indicate the flow of electrons. This is
called "electron pushing," and is how you follow reaction mechanisms in organic chemistry. The arrows begin on the electrons that are being moved, which
are usually a pair of electrons that are either currently participating in a bond, or a lone pair on an atom that are being donated to another atom to
form a new bond. The other end, the one with the arrowhead on it, is where the electrons are ending up. So if the arrow starts on a neutral oxygen and
ends up pointing to a hydrogen atom, it means the oxygen is donating its lone pair to the hydrogen to form a new bond (which usually also involves
cleaving the old bond between the hydrogen and whatever it was bonded to heterolytically). And just for future reference, there are also two kinds of arrowheads: full arrowheads and half arrowheads. A full arrowhead
means that it's a pair of electrons that are being moved; a half-sized, "hooked" arrowhead means that it's a single electron being moved. For now, we
will only be concerned with the former kind.
So with all of that in mind, let's now take a look at the acid-catalyzed reaction between acetic acid and ethanol to give ethyl acetate and water. I
have prepared the following mechanism:
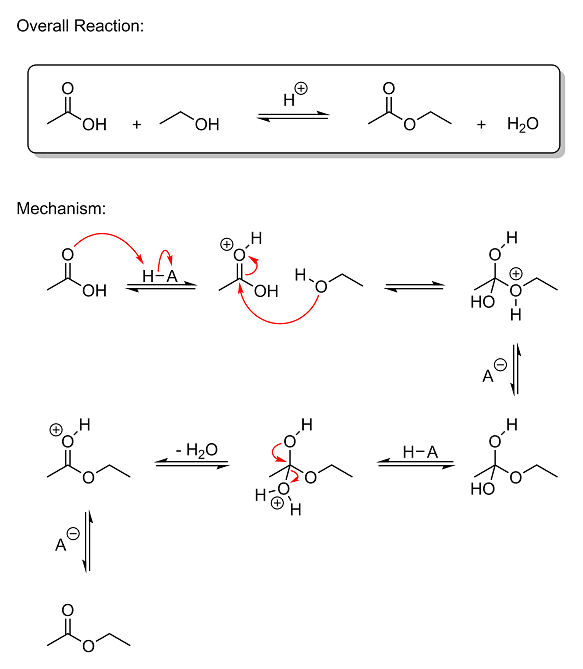
This reaction is called a Fischer esterification, and is actually rather simple. As I mentioned in your original thread back in March, this reaction
is 100% reversible, so the reactants and products are in equilibrium. The reaction is acid-catalyzed both ways, so the removal of water is necessary
to prevent protonation and hydrolysis of the ester back into a carboxylic acid.
Quick Note: the H–A in the mechanism above is the acid catalyst. The "H" represents the removable proton,
and the "A" is the rest of the molecule. The hyphen in-between them is the bond. The negatively-charged A<sup>–</sup> anion between
steps 3 and 4 and 6 and 7 is the deprotonated acid. And while I show the A<sup>–</sup> anion as being the base that removes the acidic
protons from the protonanted intermediates, in reality it could be anything with a free lone pair (like an oxygen on a water, acetic acid, ethanol, or
ethyl acetate molecule, or even an oxygen on one of the various intermediates).
So let's get started. The reaction mechanism is shown in seven steps, so I'll just go through each step one at a time:
Step 1 - In the initial step, acetic acid becomes protonated by the acid catalyst. The carbonyl oxygen (the one with the double bond)
donates its lone pair of electrons to the hydrogen atom on the acid, breaking the weak H–A bond and forming a new bond between oxygen and hydrogen.
This creates a positively-charged oxonium ion where oxygen has three bonds. One of the reasons that this happens is due to our old friend, resonance
stabilization. Remember him? By alternating the double bonds like I've shown you before, you can change which atom has the positive charge on it. Thus
the charge gets spread out across three different atoms (the carbon and both oxygens), making protonation quite favorable under acidic conditions
because the charge isn't completely localized, making it somewhat weak. Also, the reason the oxygen with the double bond gets protonated and not the
other oxygen (the hydroxyl group oxygen) is because if the other oxygen were protonated, resonance would not be able to get the positive charge off of
it. No matter what you did, you wouldn't be able to move the charge to a different atom. Not to mention that the carbon it's attached to is already
extremely electron-deficient (will explain in the next step) with a strong partial positive charge, which would create a highly unfavorable
electrostatic repulsion between the two. Thus the oxygen with the double bond is what gets protonated under strongly acidic conditions.
Step 2 - Since the carbon atom in acetic acid is bonded to two highly electronegative oxygen atoms, it naturally has a strong partial
positive charge on it; both oxygens are strongly pulling electron density away from carbon at all times. And while the partial positive on carbon is
already quite strong even without the protonation in Step 1, when that proton gets slapped onto the carbonyl oxygen, it starts pulling electron
density away from carbon FAR more strongly than it was before. Think about it: the oxygen is already highly electronegative and electron-hungry even
when it's neutral, right? So how hard do you think it's going to pull with a POSITIVE charge on it? Because now you've got a nucleus that's already
highly electronegative even when it has the same number of protons and electrons, only now that nucleus has become electron-deficient as
well. Another way to think about it is that the hydrogen is now pulling strongly on the oxygen, which, in turn, begins pulling that much harder on
carbon to make up for the loss of electron density that hydrogen is causing. This is actually an important concept in OC called the inductive effect.
And because the partial positive charge on carbon is now exceedingly strong, the carbon becomes extremely vulnerable to a nucleophilic attack by the
oxygen on ethanol. In this state, the acetic acid molecule is said to be "activated." And as such, the oxygen on ethanol, being a nucleophile (an
electron-hungry species with a free electron pair that can be donated to an electrophile), immediately attacks the positively-charged carbon atom, an
electrophile (a species with an empty orbital that attracts nucleophiles). The attack breaks the pi bond between the carbonyl oxygen and neutralizes
the positive charge by turning the carbonyl group into a hydroxyl group.
Step 3 to Step 4 - There isn't much to say about these two steps. The charged intermediate gets deprotonated by a base to give the
neutral intermediate seen in Step 4. I didn't bother to draw the arrows in these steps, but I think it's fairly obvious what happens. The acid
catalyst grabs the proton back and is regenerated. Then the bottom oxygen gets protonated by the acid to give another charged oxonium ion. The
protonation is no different than before--a lone pair on oxygen is donated to the hydrogen on the acid, breaking the H–A bond and forming a new bond
between the bottom oxygen and hydrogen. This creates the charged oxonium intermediate seen in Step 5.
Step 5 - In this step, a lone pair from the top oxygen comes down and forms a pi bond between it and carbon, breaking the bond
between carbon and the bottom oxygen. Since the bottom oxygen is positively charged, like before, it is pulling very strongly on carbon. The top
oxygen then donates a lone pair to the electron-deficient carbon, allowing the bottom oxonium group to leave as water. This is favorable because the
leaving group, water, is a neutral species. There's no charge on it to attract it back to the oppositely-charged carbon as it tries to leave. And the
protonated carbonyl group that is created when the lone pair comes down is also quite stable for the reasons mentioned in the first step (our friend,
Mr. Resonance, once again), and thus its formation is favorable.
Step 6 to Step 7 - In the final step, the protonated ester gets deprotonated by a base to give ethyl acetate.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Unfortunately, this is not a site where quality contributions get upvoted and 'good members' acquire privileges as they go along.
So all I can say is a big 'Thank you!' and that your post will be included in the upcoming new table of content.
Thanks also for spending some time on notation, that should probably have been done some time ago. I always assume that beginners are familiar with
notation from browsing chemistry web pages but I might be too optimistic on that.
|
|
|
MrHomeScientist
International Hazard
    
Posts: 1806
Registered: 24-10-2010
Location: Flerovium
Member Is Offline
Mood: No Mood
|
|
This thread is full of great information. Thanks to everyone for contributing this! This brings me back to QM in college when we went over the
hydrogen atom, and I had one of those epiphany moments where I saw how physics makes chemistry work. Amazing!
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by MrHomeScientist  | | This thread is full of great information. Thanks to everyone for contributing this! This brings me back to QM in college when we went over the
hydrogen atom, and I had one of those epiphany moments where I saw how physics makes chemistry work. Amazing! |
Yes, there can be no disputing that the central mystery that lay at the heart of chemistry, the chemical bond, could not have been solved without QM.
Thanks for the support!
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Huge Thanks Darkstar !
That is a wonderfully clear explanation of a reaction mechanism in OC.
OC seems a Lot less impenetrable now.
I'll not claim to understand it fully on 1st reading, yet it is incredibly easy to follow - thank you muchly.
Your post has been printed for proper (i.e. sober) consideration tomorrow, and probably for many days after.
Between yourself and Blogfast25 i'm actually getting educated !
Scary thought ... i could end up doing something amazing one day.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quick Navigation (Updated):
Part I – Basic Wave Mechanics
Part II - Applications of Wave Mechanics in Chemical Bond Theory
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Thanks bloggers.
I can now go to page 15 to find the index.
Darkstar's Original was a bit hard to find.
Can this be stickied in any way mods, Please ?
This is such amazingly good Chemistry/Physics Education that i absolutely refuse to see it buried in garbage posts.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  |
Can this be stickied in any way mods, Please ?
This is such amazingly good Chemistry/Physics Education that i absolutely refuse to see it buried in garbage posts. |
Thanks but only moderators have that power.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Quantum Tunnelling might be worth a try.
I'll set up a particle with a wavefunction they cannot refuse (unless they fight back with n=0).
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Quote: Originally posted by aga  | Quantum Tunnelling might be worth a try.
I'll set up a particle with a wavefunction they cannot refuse (unless they fight back with n=0). |
woelen's quite approachable. But you didn't hear that from me. Quantum entanglement will make this post re-appear elsewhere.
|
|
|
Bert
Super Administrator
        
Posts: 2821
Registered: 12-3-2004
Member Is Offline
Mood: " I think we are all going to die. I think that love is an illusion. We are flawed, my darling".
|
|
Quote: Originally posted by aga  | Quantum Tunnelling might be worth a try.
I'll set up a particle with a wavefunction they cannot refuse (unless they fight back with n=0). |
Keep yer damn tunneling wavicle in yer shorts! Were you raised in a barn, or just been hanging out with arkoma too long?!
Take a look at OP- Good enough for now?
[Edited on 31-10-2015 by Bert]
Rapopart’s Rules for critical commentary:
1. Attempt to re-express your target’s position so clearly, vividly and fairly that your target says: “Thanks, I wish I’d thought of putting it
that way.”
2. List any points of agreement (especially if they are not matters of general or widespread agreement).
3. Mention anything you have learned from your target.
4. Only then are you permitted to say so much as a word of rebuttal or criticism.
Anatol Rapoport was a Russian-born American mathematical psychologist (1911-2007).
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Brilliant ! Thank you very much Bert.
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Yep, thank you, superbert.
|
|
|
aga
Forum Drunkard
    
Posts: 7030
Registered: 25-3-2014
Member Is Offline
|
|
Appologies in advance for starting what may seem to be a polluting thread in Beginnings.
Pollution is not the intention, nor do i wish to stop learning QM here.
The Beginnings thread is merely an expression of some ideas.
|
|
|
Darkstar
Hazard to Others
  
Posts: 279
Registered: 23-11-2014
Member Is Offline
Mood: Sleepy
|
|
We will get you there eventually; however, I noticed that you said, "maybe phenyl means
phenol-shaped-bit-stuck-to-another-bit" in your competence advice thread. That is simply unacceptable. We must address this issue immediately. So it is time now for a brief introduction to
moieties and functional groups. I drew some random molecules with a bunch of functional groups on them and wrote their names next to them. I also
threw in a little refresher on skeletal structures as well. I hope you're not color blind...
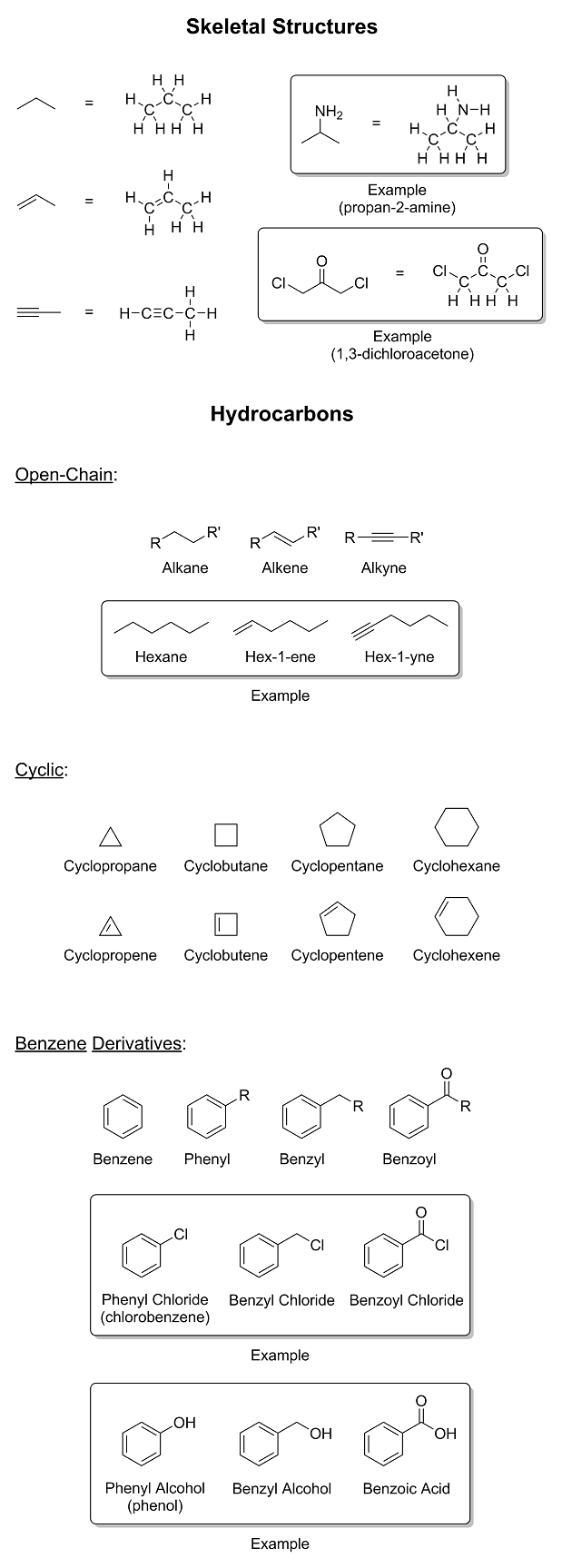
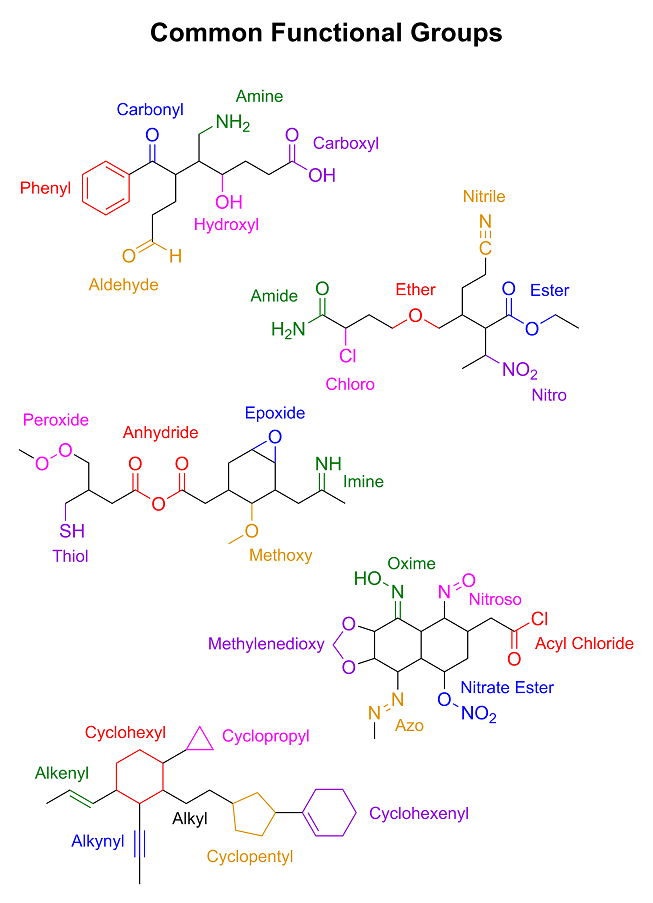
|
|
|
blogfast25
International Hazard
    
Posts: 10562
Registered: 3-2-2008
Location: Neverland
Member Is Offline
Mood: No Mood
|
|
Thanks, Darkstar. Very clear presentation.
Answers to Questions 3 and 4 tomorrow.
The questions for next week:
Question 5: (for 10 points)
Predict the geometrical shape of the following molecules:
a) Xenon difluoride, XeF<sub>2</sub>.
b) The pentafluoride xenonate anion, XeF<sub>5</sub><sup>-</sup>.
Question 6: (for 10 points)
Nitrogen difluoride (NF<sub>2</sub> has two isomers, as shown below,
left trans-NF<sub>2</sub>, right cis-NF<sub>2</sub>: has two isomers, as shown below,
left trans-NF<sub>2</sub>, right cis-NF<sub>2</sub>:
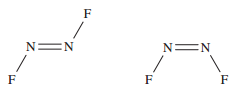
Briefly explain why two such isomers exist.
[Edited on 1-11-2015 by blogfast25]
|
|
|
| Pages:
1
..
13
14
15
16
17
..
33 |